Neutrophils initiate proinflammatory immune responses in early endometriosis lesion development
- PMID: 39836475
- PMCID: PMC11949021
- DOI: 10.1172/jci.insight.186133
Neutrophils initiate proinflammatory immune responses in early endometriosis lesion development
Abstract
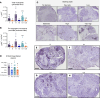
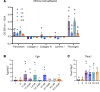
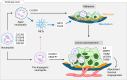
Endometriosis is a chronic gynecological disease that affects 1 in 10 reproductive-aged women. Most studies investigate established disease; however, the initiation and early events in endometriotic lesion development remain poorly understood. Our study used neutrophils from human menstrual effluent from patients with and without endometriosis for immunophenotyping, and it used a mouse model of endometriosis and a mouse endometriosis cell line to determine the role of neutrophils in the initiating events of endometriosis, including attachment and survival of minced endometrial pieces. In menstrual effluent from women with endometriosis, the ratios of aged and proangiogenic neutrophils increased compared with controls, indicating a potentially permissive proinflammatory microenvironment. In our endometriosis mouse model, knocking down neutrophil recruitment with α-CXCR2 into the peritoneum decreased endometrial tissue adhesion - supported by decreased levels of myeloperoxidase and neutrophil elastase in both developing lesions and peritoneal fluid. Fibrinogen was identified as the preferred substrate for endometrial cell adhesion in an in vitro adhesion assay and in developing lesions in vivo. Together, aged and proangiogenic neutrophils and their secretions likely promote attachment and formation of endometriotic lesions by releasing neutrophil extracellular traps and upregulating fibrinogen expression as a provisional matrix to establish attachment and survival in the development of endometriosis lesions.
Keywords: Immunology; Inflammation; Mouse models; Neutrophils; Reproductive biology.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

