Impaired axonal transport contributes to neurodegeneration in a Cre-inducible mouse model of myocilin-associated glaucoma
- PMID: 39836483
- PMCID: PMC11949003
- DOI: 10.1172/jci.insight.188710
Impaired axonal transport contributes to neurodegeneration in a Cre-inducible mouse model of myocilin-associated glaucoma
Abstract
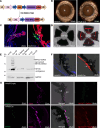
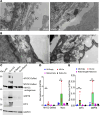
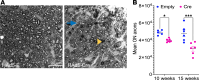
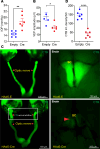
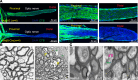
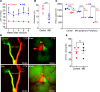
Elevation of intraocular pressure (IOP) due to trabecular meshwork (TM) dysfunction, leading to neurodegeneration, is the pathological hallmark of primary open-angle glaucoma (POAG). Impaired axonal transport is an early and critical feature of glaucomatous neurodegeneration. However, a robust mouse model that accurately replicates these human POAG features has been lacking. We report the development and characterization of a new Cre-inducible mouse model expressing a DsRed-tagged Y437H mutant of human myocilin (Tg.CreMYOCY437H). A single intravitreal injection of HAd5-Cre induced selective MYOC expression in the TM, causing TM dysfunction, reducing the outflow facility, and progressively elevating IOP in Tg.CreMYOCY437H mice. Sustained IOP elevation resulted in significant loss of retinal ganglion cells (RGCs) and progressive axonal degeneration in Cre-induced Tg.CreMYOCY437H mice. Notably, impaired anterograde axonal transport was observed at the optic nerve head before RGC degeneration, independent of age, indicating that impaired axonal transport contributes to RGC degeneration in Tg.CreMYOCY437H mice. In contrast, axonal transport remained intact in ocular hypertensive mice injected with microbeads, despite significant RGC loss. Our findings indicate that Cre-inducible Tg.CreMYOCY437H mice replicate all glaucoma phenotypes, providing an ideal model for studying early events of TM dysfunction and neuronal loss in POAG.
Keywords: Genetics; Neurodegeneration; Neuroscience; Ophthalmology; Protein misfolding; Transport.
Conflict of interest statement
Figures


Update of
-
Impaired axonal transport at the optic nerve head contributes to neurodegeneration in a novel Cre-inducible mouse model of myocilin glaucoma.bioRxiv [Preprint]. 2024 Nov 2:2024.09.18.613712. doi: 10.1101/2024.09.18.613712. bioRxiv. 2024. Update in: JCI Insight. 2025 Jan 21;10(5):e188710. doi: 10.1172/jci.insight.188710. PMID: 39345520 Free PMC article. Updated. Preprint.

