Deletion of Pyruvate Carboxylase in Tubular Epithelial Cell Promotes Renal Fibrosis by Regulating SQOR/cGAS/STING-Mediated Glycolysis
- PMID: 39836535
- PMCID: PMC11967762
- DOI: 10.1002/advs.202408753
Deletion of Pyruvate Carboxylase in Tubular Epithelial Cell Promotes Renal Fibrosis by Regulating SQOR/cGAS/STING-Mediated Glycolysis
Abstract
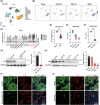
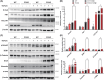
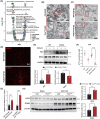
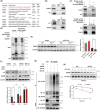
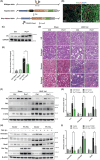
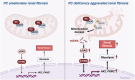
Renal fibrosis is a common pathway involved in the progression of various chronic kidney diseases to end-stage renal disease. Recent studies show that mitochondrial injury of renal tubular epithelial cells (RTECs) is a crucial pathological foundation for renal fibrosis. However, the underlying regulatory mechanisms remain unclear. Pyruvate carboxylase (PC) is a catalytic enzyme located within the mitochondria that is intricately linked with mitochondrial damage and metabolism. In the present study, the downregulation of PC in various fibrotic animal and human kidney samples is demonstrated. Renal proximal tubule-specific Pcx gene knockout mice (PcxcKO) has significant interstitial fibrosis compared to control mice, with heightened expression of extracellular matrix molecules. This is further demonstrated in a stable PC knock-out RTEC line. Mechanistically, PC deficiency reduces its interaction with sulfide:quinone oxidoreductase (SQOR), increasing the ubiquitination and degradation of SQOR. This leads to mitochondrial morphological and functional disruption, increased mtDNA release, activation of the cGAS-STING pathway, and elevated glycolysis levels, and ultimately, promotes renal fibrosis. This study investigates the molecular mechanisms through which PC deficiency induces mitochondrial injury and metabolic reprogramming in RTECs. This study provides a novel theoretical foundation and potential therapeutic targets for the pathogenesis and treatment of renal fibrosis.
Keywords: SQOR; cGAS‐STING; glycolysis; pyruvate carboxylase; renal fibrosis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rossing P., Caramori M. L., Chan J., Heerspink H., Hurst C., Khunti K., Liew A., Michos E. D., Navaneethan S. D., Olowu W. A., Sadusky T., Tandon N., Tuttle K. R., Wanner C., Wilkens K. G., Zoungas S., Craig J. C., Tunnicliffe D. J., Tonelli M. A., Cheung M., Earley A., de Boer I. H., Kidney Int. 2022, 102, 990. - PubMed
-
- Singh P., Rifkin D. E., Blantz R. C., Clin. J. Am. Soc. Nephrol. 2010, 5, 1690. - PubMed
MeSH terms
Substances
Grants and funding
- 2024PT5107/Scientific Research Program of FuRong Laboratory
- 2021Q11/Youth Science Foundation of Xiangya Hospital
- 2023QYJC031/Frontier Cross Research Project of Central South University
- 2020TQ0363/China Postdoctoral Science Foundation
- 2020M682598/China Postdoctoral Science Foundation
- 2022JJ10100/Outstanding Youth Foundation of Natural Science Foundation of Hunan Province
- 2021SK2015/Key Research and Development Program of Hunan Province
- 82090024/National Natural Science Foundation of China
- 82173877/National Natural Science Foundation of China
- 82300787/National Natural Science Foundation of China
- 82073918/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials
