4-Octyl Itaconate Alleviates Myocardial Ischemia-Reperfusion Injury Through Promoting Angiogenesis via ERK Signaling Activation
- PMID: 39836624
- PMCID: PMC11904966
- DOI: 10.1002/advs.202411554
4-Octyl Itaconate Alleviates Myocardial Ischemia-Reperfusion Injury Through Promoting Angiogenesis via ERK Signaling Activation
Abstract
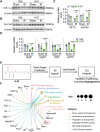
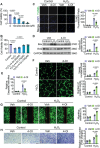
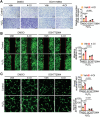
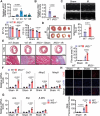
Myocardial ischemia-reperfusion (IR) injury is a critical complication following revascularization therapy for ischemic heart disease. Itaconate, a macrophage-derived metabolite, has been implicated in inflammation and metabolic regulation. This study investigates the protective role of itaconate derivatives against IR injury. Using a mice model of IR injury, the impact of 7-day 4-Octyl itaconate (4-OI) administration on cardiac function is assessed. Exogenous administration of 4-OI significantly reduces myocardial damage, enhances angiogenesis, and alleviates myocardial hypoxia injury during reperfusion. RNA sequencing and molecular docking techniques are used to find the target of itaconate, and changes in cardiac function are observed in Immune-Responsive Gene1 (IRG1) global knockout mice. In cell culture studies, 4-OI promotes endothelial cell proliferation and migration, mediated by Mitogen-Activated Protein Kinases (MAPK) signaling pathway activation, particularly through Extracellular Signal-Regulated Kinase (ERK) signaling. Inhibition of ERK blocks these beneficial effects on endothelial cells. Furthermore, itaconate synthesis inhibition worsens myocardial damage, which is mitigated by 4-OI supplementation. The results indicate that 4-OI promotes angiogenesis by activating MAPK signaling via FMS-like tyrosine kinase 1 (Flt1), highlighting its potential as a therapeutic strategy for myocardial IR injury.
Keywords: 4‐OI; IRG1; angiogenesis; myocardial ischemia‐reperfusion injury.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zweier J. L., J. Biol. Chem. 1988, 263, 1353. - PubMed
-
- Hausenloy D. J., Yellon D. M., J. Mol. Cell. Cardiol. 2003, 35, 339. - PubMed
-
- Piper H. M., Garcia‐Dorado D., Ovize M., Cardiovasc. Res. 1998, 38, 291. - PubMed
-
- Lemasters J. J., Bond J. M., Chacon E., Harper I. S., Kaplan S. H., Ohata H., Trollinger D. R., Herman B., Cascio W. E., EXS 1996, 76, 99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
