Feasibility, safety and tolerability of estrogen and/or probiotics for improving vaginal health in Canadian African, Caribbean, and Black women: A pilot phase 1 clinical trial
- PMID: 39836666
- PMCID: PMC11750099
- DOI: 10.1371/journal.pone.0315576
Feasibility, safety and tolerability of estrogen and/or probiotics for improving vaginal health in Canadian African, Caribbean, and Black women: A pilot phase 1 clinical trial
Abstract
Background: A dysbiotic vaginal microbiome (VMB) is associated with clinical conditions such as bacterial vaginosis (BV) and an increased risk of human immunodeficiency virus (HIV-1) infection. Considering the high prevalence of BV among African, Caribbean and Black (ACB) women, we conducted a prospective, randomized, open-label phase 1 clinical trial to determine the feasibility, safety and tolerability of administering low-dose estrogen, probiotics or both in combination to improve vaginal health and decrease HIV-1 susceptibility.
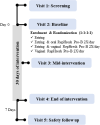
Methods: ACB women aged 18-49 from the Greater Toronto Area (GTA) were randomized to one of four study arms: intravaginal estradiol (Estring©; 7.5mg/day); a vaginal probiotic (RepHresh™ Pro-B™) administered twice daily; a combination of Estring© and vaginal RepHresh™ Pro-B™ (twice daily); or the Estring© and oral RepHresh™ Pro-B™ (twice daily), for a duration of 30 days. Feasibility was evaluated through enrolment, retention, and adherence rates, while safety and tolerability were determined by a pre- and post-treatment blood panel and reported adverse events (AEs).
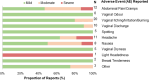
Results: Overall, 63 ACB women were screened, 50 were enrolled and received the intervention while 41 completed the study, resulting in 80% enrollment and 82% retention rates. Overall adherence to the study protocol was high at 93%, with an adherence of 92% for RepHresh™ Pro-B™ and 97% for Estring©. A total of 88 AEs were reported by 29 participants which were mild (66/88; 75%) and largely resolved (82/88;93%) by the end of the study, with no serious AEs (SAEs) noted. In addition, a panel of safety blood markers measured pre- and post-intervention confirmed no clinically significant changes in blood chemistry or blood cell count.
Conclusion: Overall, the administration of intravaginal estrogen and/or probiotics in pre-menopausal ACB women is feasible, safe, and well tolerated.
Trial registration: The trial was registered with Clinicaltrials.gov (NCT03837015) and CIHR HIV Clinical Trials (CTN308).
Copyright: © 2025 Gill et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: GR developed the probiotic strains GR-1 and RC-14 but has had no financial interest in them for 15 years. GR consults for Seed, a company producing probiotic strains not used in this study. He was not paid salary for any roles with the company. All the other authors have declared no conflict of interest. This does not alter our adherence to PLOS One policies on sharing materials and data.”
Figures



Similar articles
-
Effect of the vaginal live biotherapeutic LACTIN-V (Lactobacillus crispatus CTV-05) on vaginal microbiota and genital tract inflammation among women at high risk of HIV acquisition in South Africa: a phase 2, randomised, placebo-controlled trial.Lancet Microbe. 2025 Jun;6(6):101037. doi: 10.1016/j.lanmic.2024.101037. Epub 2025 Apr 4. Lancet Microbe. 2025. PMID: 40194532 Free PMC article. Clinical Trial.
-
Effects of Pro/Prebiotics Alone over Pro/Prebiotics Combined with Conventional Antibiotic Therapy to Treat Bacterial Vaginosis: A Systematic Review.Int J Clin Pract. 2022 Apr 21;2022:4774783. doi: 10.1155/2022/4774783. eCollection 2022. Int J Clin Pract. 2022. PMID: 35685517 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD010503. doi: 10.1002/14651858.CD010503.pub2. Cochrane Database Syst Rev. 2017. PMID: 28257559 Free PMC article.
References
-
- Fu L, Sun Y, Han M, Wang B, Xiao F, Zhou Y, et al.. Incidence trends of five common sexually transmitted infections excluding HIV from 1990 to 2019 at the global, regional, and national levels: results from the global burden of disease Study 2019. Front Med. 2022. Mar 2;9:851635. doi: 10.3389/fmed.2022.851635 . - DOI - PMC - PubMed
-
- Logie CH, Jenkinson JIR, Earnshaw V, Tharao W, Loutfy MR. A structural equation model of HIV-related stigma, racial discrimination, housing insecurity and wellbeing among African and Caribbean Black women living with HIV in Ontario, Canada. PLoS One. 2016. Sep 1;11(9):e0162826. doi: 10.1371/journal.pone.0162826 . - DOI - PMC - PubMed
-
- Etowa J, Tharao W, Mbuagbaw L, Baidoobonso S, Hyman I, Obiorah S, et al.. Community perspectives on addressing and responding to HIV-testing, pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) among African, Caribbean and Black (ACB) people in Ontario, Canada. BMC Public Health. 2022. Dec 1;22(1):913. doi: 10.1186/s12889-022-13093-0 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

