Persistently increased post-stress activity of paraventricular thalamic neurons is essential for the emergence of stress-induced alterations in behaviour
- PMID: 39836670
- PMCID: PMC11750107
- DOI: 10.1371/journal.pbio.3002962
Persistently increased post-stress activity of paraventricular thalamic neurons is essential for the emergence of stress-induced alterations in behaviour
Abstract
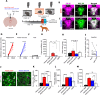
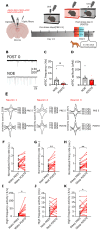
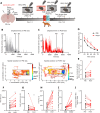
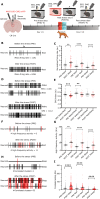
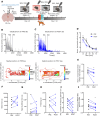
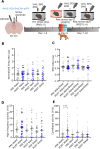
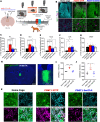
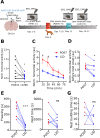
A single exposure to a stressful event can result in enduring changes in behaviour. Long-term modifications in neuronal networks induced by stress are well explored but the initial steps leading to these alterations remain incompletely understood. In this study, we found that acute stress exposure triggers an immediate increase in the firing activity of calretinin-positive neurons in the paraventricular thalamic nucleus (PVT/CR+) that persists for several days in mice. This increase in activity had a causal role in stress-induced changes in spontaneous behaviour. Attenuating PVT/CR+ neuronal activity for only 1 h after the stress event rescued both the protracted increase in PVT/CR+ firing rate and the stress-induced behavioural alterations. Activation of the key forebrain targets (basolateral amygdala, prelimbic cortex, and nucleus accumbens) that mediate defensive behaviour has also been reduced by this post-stress inhibition. Reduction of PVT/CR+ cell activity 5 days later remained still effective in ameliorating stress-induced changes in spontaneous behaviour. The results demonstrate a critical role of the prolonged, post-stress changes in firing activity of PVT/CR+ neurons in shaping the behavioural changes associated with stress. Our data proposes a therapeutic window for intervention in acute stress-related disorders, offering potential avenues for targeted treatment strategies.
Copyright: © 2025 Jász et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

