Gingipains protect Porphyromonas gingivalis from macrophage-mediated phagocytic clearance
- PMID: 39836688
- PMCID: PMC11801703
- DOI: 10.1371/journal.ppat.1012821
Gingipains protect Porphyromonas gingivalis from macrophage-mediated phagocytic clearance
Abstract
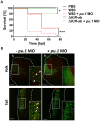
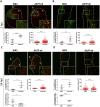
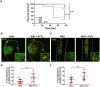
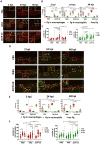
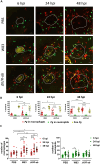
Porphyromonas gingivalis (Pg) is a keystone pathogen in periodontitis, a highly prevalent disease manifested by chronic inflammation of the periodontium, alveolar bone resorption and tooth loss. During periodontitis pathobionts such as Pg can enter the bloodstream and growing evidence correlates periodontitis with increased risk of cardiovascular and neurodegenerative diseases. However, the mechanism by which immune cells respond to Pg challenge in vivo remains elusive. Pg produce aggressive proteolytic virulence factors termed gingipains which not only provide nutrients necessary for bacterial growth, but also subvert the host immune response, facilitating bacterial survival. Using transgenic zebrafish with fluorescently labelled macrophages and neutrophils, the role of gingipains in bacterial survival and interaction with phagocytes during systemic and local infection was examined. In contrast to the wild-type (W83) Pg, isogenic gingipain-null (ΔK/R-ab) or wild-type Pg treated with gingipain inhibitors caused less zebrafish mortality, bacteria were rapidly phagocytosed, acidified in phagosomes and eradicated when systemically injected, showing that gingipains are instrumental in preventing phagocytosis and intracellular killing of Pg by innate immune cells. Moreover, Pg were predominantly phagocytosed by macrophages, and gingipain depletion/inactivation increased bacterial phagocytosis when bacteria were injected either systemically or locally in the otic vesicle, with less bacteria internalised by neutrophils. This phenomenon was Pg-specific as Fusobacterium nucleatum caused neutrophil recruitment that then effectively phagocytosed these bacteria. These data demonstrate the important role of phagocytes, especially macrophages, in combating Pg infection and highlight the crucial protective role of gingipains in subverting the innate immune response. This study also emphasizes the advantages of using zebrafish to study interactions of Pg with phagocytes in vivo in real-time, providing a valuable experimental system for testing new therapeutic strategies aimed at reducing periodontal-associated systemic or neurodegenerative disease.
Copyright: © 2025 Widziolek et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

