Balancing central control and sensory feedback produces adaptable and robust locomotor patterns in a spiking, neuromechanical model of the salamander spinal cord
- PMID: 39836708
- PMCID: PMC11771899
- DOI: 10.1371/journal.pcbi.1012101
Balancing central control and sensory feedback produces adaptable and robust locomotor patterns in a spiking, neuromechanical model of the salamander spinal cord
Abstract
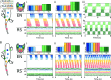
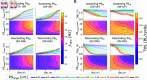
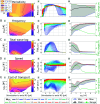
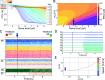
This study introduces a novel neuromechanical model employing a detailed spiking neural network to explore the role of axial proprioceptive sensory feedback, namely stretch feedback, in salamander locomotion. Unlike previous studies that often oversimplified the dynamics of the locomotor networks, our model includes detailed simulations of the classes of neurons that are considered responsible for generating movement patterns. The locomotor circuits, modeled as a spiking neural network of adaptive leaky integrate-and-fire neurons, are coupled to a three-dimensional mechanical model of a salamander with realistic physical parameters and simulated muscles. In open-loop simulations (i.e., without sensory feedback), the model replicates locomotor patterns observed in-vitro and in-vivo for swimming and trotting gaits. Additionally, a modular descending reticulospinal drive to the central pattern generation network allows to accurately control the activation, frequency and phase relationship of the different sections of the limb and axial circuits. In closed-loop swimming simulations (i.e. including axial stretch feedback), systematic evaluations reveal that intermediate values of feedback strength increase the tail beat frequency and reduce the intersegmental phase lag, contributing to a more coordinated, faster and energy-efficient locomotion. Interestingly, the result is conserved across different feedback topologies (ascending or descending, excitatory or inhibitory), suggesting that it may be an inherent property of axial proprioception. Moreover, intermediate feedback strengths expand the stability region of the network, enhancing its tolerance to a wider range of descending drives, internal parameters' modifications and noise levels. Conversely, high values of feedback strength lead to a loss of controllability of the network and a degradation of its locomotor performance. Overall, this study highlights the beneficial role of proprioception in generating, modulating and stabilizing locomotion patterns, provided that it does not excessively override centrally-generated locomotor rhythms. This work also underscores the critical role of detailed, biologically-realistic neural networks to improve our understanding of vertebrate locomotion.
Copyright: © 2025 Pazzaglia et al. This is an open access article distributed under the terms of the CreativeCommonsAttributionLicense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
A new model of the spinal locomotor networks of a salamander and its properties.Biol Cybern. 2018 Aug;112(4):369-385. doi: 10.1007/s00422-018-0759-9. Epub 2018 May 22. Biol Cybern. 2018. PMID: 29790009
-
Modeling spinal locomotor circuits for movements in developing zebrafish.Elife. 2021 Sep 2;10:e67453. doi: 10.7554/eLife.67453. Elife. 2021. PMID: 34473059 Free PMC article.
-
A salamander's flexible spinal network for locomotion, modeled at two levels of abstraction.Integr Comp Biol. 2013 Aug;53(2):269-82. doi: 10.1093/icb/ict067. Epub 2013 Jun 18. Integr Comp Biol. 2013. PMID: 23784700
-
Decoding the mechanisms of gait generation in salamanders by combining neurobiology, modeling and robotics.Biol Cybern. 2013 Oct;107(5):545-64. doi: 10.1007/s00422-012-0543-1. Epub 2013 Feb 22. Biol Cybern. 2013. PMID: 23430277 Review.
-
Origin of excitatory drive to a spinal locomotor network.Brain Res Rev. 2008 Jan;57(1):22-8. doi: 10.1016/j.brainresrev.2007.06.015. Epub 2007 Jul 27. Brain Res Rev. 2008. PMID: 17825424 Review.
Cited by
-
FARMS: Framework for Animal and Robot Modeling and Simulation.bioRxiv [Preprint]. 2025 Feb 21:2023.09.25.559130. doi: 10.1101/2023.09.25.559130. bioRxiv. 2025. PMID: 38293071 Free PMC article. Preprint.
References
MeSH terms
LinkOut - more resources
Full Text Sources

