Clinical Validation of MyProstateScore 2.0 Testing Using First-Catch, Non-Digital Rectal Examination Urine
- PMID: 39836866
- PMCID: PMC11981841
- DOI: 10.1097/JU.0000000000004421
Clinical Validation of MyProstateScore 2.0 Testing Using First-Catch, Non-Digital Rectal Examination Urine
Abstract
Purpose: The 18-gene MyProstateScore 2.0 (MPS2) test was previously validated for detection of grade group (GG) ≥ 2 prostate cancer using post-digital rectal examination (DRE) urine. To improve ease of testing, we validated MPS2 using first-catch, non-DRE urine.
Materials and methods: Patients provided first-catch urine before biopsy. MPS2 values were calculated using previously validated models differing only by extent of clinical data included biomarkers alone (BA; no clinical data), biomarkers and clinical factors (BA + CF), and biomarkers, clinical factors, and prostate volume (BA + CF + PV). The primary outcome was GG ≥ 2 cancer on biopsy. MPS2 performance and clinical consequences of testing were compared with PSA and the Prostate Cancer Prevention Trial risk calculator (PCPTrc).
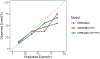
Results: The cohort included 266 men with median PSA 6.6 ng/mL (IQR, 4.9-9.1) of whom 103 (39%) had GG ≥ 2 cancer on biopsy. The AUC for GG ≥ 2 cancer was 57% for PSA, 62% for PCPTrc, and 71%, 74%, and 77% for MPS2 models. Under a testing approach detecting > 90% of GG ≥ 2 cancers, MPS2 testing would have avoided 36% to 42% of unnecessary biopsies, as compared with 13% using the PCPTrc. In patients with a prior negative biopsy, MPS2 testing would have avoided 44% to 53% of repeat biopsies, as compared with only 2.6% using PCPTrc.
Conclusions: Using first-catch urine, MPS2 meaningfully improved the proportion of biopsies avoided relative to PCPTrc while maintaining highly sensitive detection of GG ≥ 2 cancer. Non-DRE testing provides a convenient, objective, and highly accurate testing option to reduce the need for imaging and biopsy in men with elevated PSA.
Keywords: biomarkers; early detection of cancer; liquid biopsy; prostate cancer.
Figures
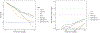
Comment in
-
Editorial Comment.J Urol. 2025 May;213(5):589. doi: 10.1097/JU.0000000000004445. Epub 2025 Jan 31. J Urol. 2025. PMID: 39886940 No abstract available.
References
-
- National Comprehensive Cancer Network. Prostate cancer early detection (version 2.2024). Accessed July 31, 2024. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

