Peripheral blood age-sensitive immune markers in multiple sclerosis: relation to sex, cytomegalovirus status, and treatment
- PMID: 39837012
- PMCID: PMC11788784
- DOI: 10.1016/j.ebiom.2025.105559
Peripheral blood age-sensitive immune markers in multiple sclerosis: relation to sex, cytomegalovirus status, and treatment
Abstract
Background: Immunosenescence is accelerated by chronic infectious and autoimmune diseases and could contribute to the pathobiology of multiple sclerosis (MS). How MS and disease-modifying therapies (DMTs) impact age-sensitive immune biomarkers is only partially understood.
Methods: We analyzed 771 serum samples from 147 healthy controls and 289 people with MS (PwMS) by multiplex immunoassays. We determined cytomegalovirus (CMV) serostatus and collected retrospective clinical information. We performed unsupervised and multivariable analyses.
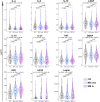
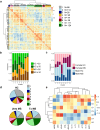
Findings: Unsupervised analyses revealed that MS immune profile was characterized by low relative levels of anti-inflammatory/neuroprotective factors IL-4, IL-10, TNF, and β-NGF but high levels of growth factors EGF and bFGF. Serum levels of IL-4, β-NGF, IL-27, BDNF, and leptin were significantly influenced by sex and/or CMV status. IL-4 and β-NGF levels were lower in untreated PwMS compared to controls, while EGF and bFGF levels were influenced by age and markedly elevated in PwMS in multivariable analysis. Samples from treated PwMS, but not untreated PwMS, showed lower levels of BDNF and TNF than controls. Initiation of high efficacy DMTs, but not low efficacy DMTs, was associated with reduced levels of bFGF and EGF. Samples associated with distinct DMTs exhibited specific profiles for age-sensitive immune markers. Finally, lower levels of IL-6, TNF, IL-10, and β-NGF were observed at baseline in PwMS who subsequently experienced clinical failure after DMTs initiation.
Interpretation: Age, sex, CMV status, and specific DMTs significantly influence levels of age-sensitive immune biomarkers associated with MS and must be considered when investigating inflammation-related biomarkers.
Funding: This work was supported by a Grant for Multiple Sclerosis Innovation by Merck KGaA (ID: 10.12039/100009945).
Keywords: Aging; Autoimmunity; Central nervous system; Immunosenescence; Neurological disease.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests H.D. received a postdoctoral award from the National Multiple Sclerosis Society. R.B. and M.L.C. received a doctoral award from MS Canada. P.D. served on editorial boards and has been supported to attend meetings by EMD, Biogen, Novartis, Genzyme, and TEVA Neuroscience. He holds grants from the CIHR and the MS Society of Canada and has received funding for investigator-initiated trials from Biogen, Novartis, and Genzyme. A.P. holds the Senior Canada Research Chair in Multiple Sclerosis and active patents. WO2016095046A1, US20110014183A1, and US20100310568A1; has served sporadically on scientific advisory boards or as a speaker for Novartis, Biogen, Sanofi, Bristol Myers Squibb, Actelion, Roche, and EMD-Serono. G.M. has received an Innovations in Well-Being Award for National MS Society/International Progressive Multiple Sclerosis Alliance (grant #PA-2304-41062) (2024–2025), has participated in Advisory boards for Novartis, Merck/EMD Serono and Genentech-Roche, and educational programs for John Hopkins e-Literature review, Neurology Live, MedEdge, BIogen and Novartis. C.L. is an FRQS Clinicien–Chercheur Junior 2 Scholar; has served sporadically on scientific advisory boards or as a speaker for FIND Therapeutics, Amgen, Novartis, Biogen, Sanofi, Bristol Myers Squibb, Actelion, Roche, and EMD Inc. Mississauga an affiliate of Merck KGaA. All other authors report no conflicts of interest relevant to this article.
Figures


References
-
- Samjoo I.A., Worthington E., Drudge C., et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10(6):495–507. - PubMed
-
- Larochelle C., Uphaus T., Prat A., Zipp F. Secondary progression in multiple sclerosis: neuronal exhaustion or distinct pathology? Trends Neurosci. 2016;39(5):325–339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

