Treating Hematological Malignancies With OR-2100, an Orally Bioavailable Prodrug of Decitabine
- PMID: 39837580
- PMCID: PMC11967254
- DOI: 10.1111/cas.16452
Treating Hematological Malignancies With OR-2100, an Orally Bioavailable Prodrug of Decitabine
Erratum in
-
Correction to "Treating Hematological Malignancies With OR-2100, an Orally Bioavailable Prodrug of Decitabine".Cancer Sci. 2025 Oct 20. doi: 10.1111/cas.70230. Online ahead of print. Cancer Sci. 2025. PMID: 41116325 No abstract available.
Abstract
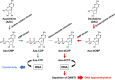
DNA methylation is an enzyme-driven epigenetic modification that must be precisely regulated to maintain cellular homeostasis. Aberrant methylation status, especially hypermethylation of the promoter sites of tumor-suppressor genes, is observed in human malignancies and is a proven target for cancer therapy. The first-generation DNA demethylating agents, azacitidine and decitabine, are widely used for treating several hematological malignancies. In addition, orally bioavailable prodrugs of azacitidine and decitabine have recently been approved by the FDA. We have developed a silylated derivative of decitabine, OR-2100, which is resistant to degradation by cytidine deaminase and orally bioavailable. It has efficacy against several human hematological malignancies in xenograft mouse models with less hematotoxicity than decitabine. Since DNA demethylating agents are combined with molecularly targeted drugs in clinical use and trials, we think that the less hematotoxic profile of OR-2100 makes it suitable for use as a combination therapy. In this article, we review the therapeutic approach in hematological malignancies with the DNA demethylating agent OR-2100.
Keywords: DNA demethylating agents; DNA methylation; combination therapy; hematological malignancies; prodrug.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
S.K. has received honoraria from Bristol‐Myers Squibb, Novartis, Pfizer, and Otsuka Pharmaceuticals, and research funding from Bristol‐Myers Squibb, Novartis, Pfizer, and Otsuka Pharmaceuticals, and OHARA Pharmaceutical. The other authors declare they have no potential conflicts of interest. S.K. is an editorial board member of Cancer Science.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

