Renal anemia and hyporesponsiveness to ESA for preservation of residual kidney function in patients undergoing peritoneal dialysis
- PMID: 39838061
- PMCID: PMC11751488
- DOI: 10.1038/s41598-025-87456-z
Renal anemia and hyporesponsiveness to ESA for preservation of residual kidney function in patients undergoing peritoneal dialysis
Abstract
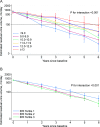
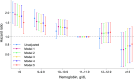
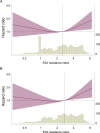
Preservation of residual kidney function (RKF) is important in patients undergoing peritoneal dialysis (PD). We aimed to examine the association between anemia management and residual urine output using data from a nationwide survey of dialysis patients. After excluding patients with anuria at baseline from the Total cohort of 2,712, 659 of 1,640 patients developed anuria during a median follow-up of 2.5 (interquartile range: 1.5-4.2) years. Urine volume decreased more rapidly as hemoglobin decreased or as the erythropoiesis-stimulating agent (ESA) resistance index (ERI) increased. The hazard ratios with 95% confidence intervals for the development of anuria, defined as residual urine volume ≤ 100 mL/day, were 1.65 (1.20-2.27), 1.39 (1.08-1.77), and 1.32 (1.07-1.63) for hemoglobin levels of < 9.0, 9.0-9.9, and 10.0-10.9 g/dL compared with 11.0-11.9 g/dL, and 1.35 (1.10-1.66) and 1.41 (1.14-1.73) for the second and third tertiles of ERI compared with the first tertile. In conclusion, patients with a low hemoglobin level or a high ERI were more likely to experience a decline in residual urine output and to develop anuria. Further studies are needed to investigate the effects of interventions that could improve renal anemia and/or ESA hyporesponsiveness on RKF preservation.
Keywords: Anuria; Erythropoiesis-stimulating agent resistance index; Hyporesponsiveness to erythropoiesis-stimulating agent; Peritoneal dialysis; Residual kidney function.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TI received a research grant from Kyowa Kirin Co., Ltd. and a consultation fee from GlaxoSmithKline. TH received a research grant from JSPS KAKENHI (Grant Number 19K03092 and 24K06239), and honoraria from Kyowa Kirin, Baxter, Terumo, and Torii Pharmaceutical. TKo received honoraria from Kowa, Torii, Sanwa Kagaku Kenkyusyo, and AstraZeneca. HN received speaker honoraria from Kyowa Kirin, Baxter, Terumo, Ono Pharmaceutical Co. Ltd., Daiichi-Sankyo, and Kissei Pharmaceutical Co. Ltd. MA received honoraria from Kyowa Kirin, Baxter, Terumo, Mitsubishi Tanabe, Torii, Bayer Yakuhin, and Astellas. HH is a scientific advisor for Astellas Pharma, Bayer Yakuhin, Kyowa Kirin, Mitsubishi Tanabe Pharma, and Torii Pharmaceutical and has obtained research funds from Chugai Pharmaceutical, Kyowa Kirin, Otsuka Pharmaceutical, and Torii Pharmaceutical and lecture fees from Astellas Pharma, Bayer Yakuhin, Chugai Pharmaceutical, Kissei Pharmaceutical, Kyowa Kirin, Mitsubishi Tanabe Pharma, and Torii Pharmaceutical. KT received honoraria from Kyowa Kirin, Mitsubishi-Tanabe, Astellas, Torii, Bayer, Baxter, Kissei, and Chugai and endorsements from Kyowa Kirin, Baxter, Terumo, Torii, Chugai, and Bayer. YI received research grants and speaker honoraria from Chugai Pharmaceutical, Kyowa Kirin, Astellas Pharma, Mitsubishi Tanabe Pharma, Bayer, and Torii Pharmaceuticals. TKu received research grants from Ono Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., speaker bureaus from Kyowa Kirin, Fuso Pharmaceutical Industries, Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., AstraZeneca Plc., and Bayer Yakuhin Ltd. All the remaining authors have nothing to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

