Efficacy and safety of gut microbiome-targeted treatment in patients with depression: a systematic review and meta-analysis
- PMID: 39838303
- PMCID: PMC11753086
- DOI: 10.1186/s12888-024-06438-z
Efficacy and safety of gut microbiome-targeted treatment in patients with depression: a systematic review and meta-analysis
Abstract
Background: The study aimed to comprehensively analyze and establish a framework for evaluating the efficacy of microbiome-targeted treatment (MTT) for depression.
Methods: We searched PubMed, Embase, Cochrane Library, Web of Science, and the Chinese National Knowledge Infrastructure database for randomized controlled trials (RCTs) on MTT in treating depression until October 19, 2023. A meta-analysis was conducted to evaluate the efficacy and safety of MTT. Comprehensive subgroup analyses were undertaken to explore factors influencing MTT's efficacy in treating depression. This study was registered with PROSPERO (CRD42023483649).
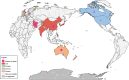
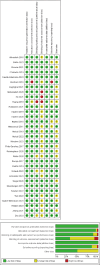
Results: The study selection process identified 51,570 studies, of which 34 met the inclusion criteria. The overall pooled estimates showed that MTT significantly improved depression symptoms (SMD -0.26, 95% CI [-0.32, -0.19], I2 = 54%) with acceptable safety. Subgroup analyses by geography showed that effectiveness was demonstrated in Asia (SMD -0.46, 95% CI [-0.56, -0.36], I2 = 36%), while no evidence of effectiveness was found in Europe (SMD -0.07, 95% CI [-0.19, 0.05], I2 = 55%), America (SMD -0.33, 95% CI [-0.67, 0.02], I2 = 60%), and Oceania (SMD 0.00, 95% CI [-0.18, 0.18], I2 = 0%). Besides, the efficacy was shown in depressed patients without comorbidities (SMD -0.31, 95% CI [-0.40, -0.22], I2 = 0%), whereas effectiveness was poor in those with digestive disorders, such as irritable bowel syndrome (SMD -0.37, 95% CI [-0.89, 0.16], I2 = 74%), chronic diarrhea (SMD -0.34, 95% CI [-0.73, 0.05]), and chronic constipation (SMD -0.23, 95% CI [-0.57, 0.11], I2 = 0%). In perinatal depressed patients, MTT was not effective (SMD 0.16, 95% CI [0.01, 0.31], I2 = 0%). It was found that < 8 weeks (SMD -0.33, 95% CI [-0.45, -0.22], I2 = 0%) and 8-12 weeks (SMD -0.34, 95% CI [-0.44, -0.23], I2 = 57%) MTT were effective, while > 12 weeks (SMD 0.02, 95% CI [-0.12, 0.17], I2 = 68%) MTT was ineffective.
Conclusions: Despite the overall effectiveness of MTT in treating depression and its acceptable safety profile, caution is warranted in drawing this conclusion due to limitations posed by the small sample size of included studies and heterogeneity. The efficacy of MTT for depression exhibits variation influenced by geography, patient comorbidities, and duration of administration.
Keywords: Depression; Geographical region; Meta-analysis; Microbiome–targeted therapy; Patient characteristics; Precision medicine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
A meta-analysis of randomized controlled trials evaluating the effectiveness of fecal microbiota transplantation for patients with irritable bowel syndrome.BMC Gastroenterol. 2024 Jul 5;24(1):217. doi: 10.1186/s12876-024-03311-x. BMC Gastroenterol. 2024. PMID: 38970007 Free PMC article.
-
Efficacy and safety of Shouhui Tongbian Capsules in the treatment of constipation: A systematic review and meta-analysis.Phytomedicine. 2023 Jan;108:154541. doi: 10.1016/j.phymed.2022.154541. Epub 2022 Nov 7. Phytomedicine. 2023. PMID: 36375236
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
-
Meta-analysis of randomized controlled trials of the effects of probiotics in Parkinson's disease.Food Funct. 2023 Apr 24;14(8):3406-3422. doi: 10.1039/d2fo03825k. Food Funct. 2023. PMID: 36974511 Review.
References
-
- Friedrich MJ. Depression is the leading cause of disability around the world[J]. JAMA. 2017;317(15):1517. - PubMed
-
- Shapira I, Burk BG, Hill H, et al. Playing with a full dex of cards: treatment resistant depression with suicidality responds to inpatient dextromethorphan therapy[J]. Psychiatry Res Case Rep. 2023;2(1):100105.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

