Robust circulating microRNA signature for the diagnosis and early detection of pancreatobiliary cancer
- PMID: 39838364
- PMCID: PMC11752661
- DOI: 10.1186/s12916-025-03849-x
Robust circulating microRNA signature for the diagnosis and early detection of pancreatobiliary cancer
Erratum in
-
Correction: Robust circulating microRNA signature for the diagnosis and early detection of pancreatobiliary cancer.BMC Med. 2025 Apr 16;23(1):225. doi: 10.1186/s12916-025-04079-x. BMC Med. 2025. PMID: 40241086 Free PMC article. No abstract available.
Abstract
Background: A new circulating biomarker superior to carbohydrate antigen 19-9 (CA19-9) is needed for diagnosing pancreatobiliary cancer (PBca). The aim of this study was to identify serum microRNA (miRNA) signatures comprising reproducible and disease-related miRNAs.
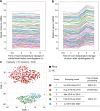
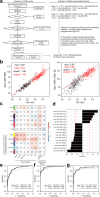
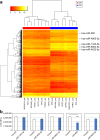
Methods: This multicenter study involved patients with treatment-naïve PBca and healthy participants. The optimized serum processing conditions were evaluated using t-distributed stochastic neighbor embedding (t-SNE) visualization. Serum miRNA candidates for disease association were selected using weighted gene coexpression network analysis (WGCNA). A miRNA signature combining multiple serum miRNAs was tested in exploratory, validation, and independent validation sets. The synthesis and secretion of diagnostic miRNAs were evaluated using human pancreatic cancer cells.
Results: In total, 284 (150 healthy and 134 PBca) of 827 serum samples were processed within 2 h of blood collection before freezing, distributed in the same area as that in the t-SNE map, and assigned to an exploratory set. The 193 optimized samples were assigned to either the validation (50 healthy, 47 PBca) or independent validation (50 healthy, 46 PBca) set. Index-1, a combination of five serum miRNAs (hsa-miR-1343-5p, hsa-miR-4632-5p, hsa-miR-4665-5p, hsa-miR-665, and hsa-miR-6803-5p) with disease association in WGCNA, showed a sensitivity and specificity of > 80% and an AUC outperforming that of CA19-9 in the exploratory, validation, and independent validation sets. The AUC of Index-1 was superior to that of CA19-9 (0.856 vs. 0.649, p = 0.038) for detecting T1 tumors. miR-665, a component of Index-1, was expressed in human pancreatic cancer cells, and its transfection inhibited cell growth.
Conclusions: The serum miRNA signature Index-1 is useful for detecting PBca and could facilitate the early diagnosis of PBca. These findings can help improve clinical PBca detection by providing an optimized biomarker that overcomes the limitations of the current standard.
Keywords: Biomarker; Circulating microRNA; Pancreatobiliary cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This prospective observational study was approved by the ethics review committee of the National Cancer Center Hospital East and National Cancer Center Hospital (approval no. 2016–049, 2020–449) and the institutional review boards of collaborating institutions. The study for time-course effects was approved by the Human Tissue Samples Ethics Committee for R&D of Toray Industries, Inc. (HC2017-10, HC2021-3, and HC2021-18). All the studies complied with the principles of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Written informed consent was obtained from all participants. Consent for publication: Not applicable. Competing interests: S.M. received honoraria and grants from Toray Industries, Inc., and grants from the Japan Agency for Medical Research and Development (AMED) under Grant Numbers 15ck0106027h0002 and 16ck106027h0003 during the submitted work, as well as research funding from Ajinomoto Co., Inc., PFDeNA Co., Ltd., and Mitsui Chemicals Inc. outside the submitted work. M.I. received research funding from Astellas Pharma Inc., Nihon Servier Co., Ltd., and Novartis Pharma K.K. and received honoraria from Guardant Health Japan Corp., Nihon Servier Co., Ltd., Taiho Pharmaceutical Co., Ltd., M.S.D. K.K., NIPPON KAYAKU Co., Ltd., Yakult Honsha Co., Ltd., Boehringer Ingelheim GmbH, D.F.P. (Delta Fly Pharma) Inc., Merus N.V., Invitae Corp., and Novartis Pharma K.K. outside the submitted work. M.U. received grants from Toray Industries, Inc., during the submitted work, as well as research funding from Taiho Pharmaceutical Co., Ltd., AstraZeneca K.K., M.S.D. K.K., Nihon Servier Co., Ltd., Ono Pharmaceutical Co., Ltd., Incyte Biosciences Japan G.K., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim GmbH, Eisai Co., Ltd., Novartis Pharma K.K., Astellas Pharma Inc., J-pharma Co., Ltd., DFP (Delta Fly Pharma) Inc., Novocure GmbH, and Chiome Bioscience Inc. and honoraria from Taiho Pharmaceutical Co., Ltd., AstraZeneca K.K., Yakult Honsha Co., Ltd., M.S.D. K.K., Nihon Servier Co., Ltd., Ono Pharmaceutical Co., Ltd., Incyte Biosciences Japan G.K., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim GmbH, J-pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Novartis Pharma K.K. outside the submitted work. K.H2. received honoraria and research funding from Toray Industries, Inc., during the submitted work. K.T. received research funding from Toray Industries, Inc., during the submitted work, received research funding from Hitachi High-Tech Corporation and H.U. Group Research Institute G.K., and holds a patent with H.U. Group Research Institute G.K. outside the submitted work. M.T., I.M., T.K., H.Y., H.I1, H.I2, E.H., K.N., M.N., and A.O. received research funding from Toray Industries, Inc., during the submitted work. H.S. is an employee of Toray Industries, Inc., and received grants from AMED under grant numbers 15ck0106027h0002 and 16ck106027h0003 during the submitted work. S.T. is an employee of Toray Industries, Inc., and received grants from AMED under grant numbers 15ck0106027h0002 and 16ck106027h0003 during the submitted work. The other authors declare that they have no competing interests.
Figures



References
-
- National Cancer Center. Cancer registry and statistics. Cancer Information Service, National Cancer Center. http://ganjoho.jp/public/statistics/pub/statistics01.html. Accessed 27 Sep 2023. [Japanese].
-
- Mancuso A, Calabrò F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–41. - PubMed
-
- Mitsunaga S. Transition of patient background and treatment outcomes for bile duct cancer – a study of 1031 cases at the National Cancer Center East Hospital. The Japanese Society of Medical Oncology Annual Meeting; 2013/08/29–2013/08/31. p. O1–47. [Japanese].
-
- The Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. The 2019 revision of the Clinical Practice Guidelines for pancreatic cancer; 2019. [Japanese]. https://www.suizou.org/pdf/pancreatic_cancer_cpg-2019.pdf
-
- Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci. 2019;28:26–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

