Exploring the ability of plasma pTau217, pTau181 and beta-amyloid in mirroring cerebrospinal fluid biomarker profile of Mild Cognitive Impairment by the fully automated Lumipulse® platform
- PMID: 39838411
- PMCID: PMC11748262
- DOI: 10.1186/s12987-025-00620-5
Exploring the ability of plasma pTau217, pTau181 and beta-amyloid in mirroring cerebrospinal fluid biomarker profile of Mild Cognitive Impairment by the fully automated Lumipulse® platform
Abstract
Background: The approval of new disease-modifying therapies by the U.S. Food and Drug Administration and the European Medicine Agency makes it necessary to optimize non-invasive and cost-effective tools for the identification of subjects at-risk of developing Alzheimer's Disease (AD). Plasma biomarkers are excellent candidates. However, their ability to reflect the cerebrospinal fluid (CSF) profile - that remains to date the gold standard for the biochemical diagnosis of AD - needs to be confirmed and validated before their implementation in clinical practice. The aims of this study are to analyse the correlation between CSF and plasma Aβ40, Aβ42, Aβ42/Aβ40 and pTau181, and to assess the diagnostic performance of plasma biomarkers in a cohort of subjects affected by Mild Cognitive Impairment (MCI).
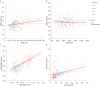
Methods: The study was performed on 306 subjects affected by MCI, enrolled in the context of the Italian Interceptor Project. Aβ40, Aβ42 and pTau181 were analysed in plasma and CSF, and pTau217 was measured in plasma. The fully automated chemiluminescence enzyme immunoassay and the Lumipulse® G600II (Fujirebio) instrument were used for all measurements. We analysed the correlations between CSF and plasma biomarkers and the differences of plasma biomarker concentrations after grouping MCI cases according to AT classification of CSF AD biomarker profiles.
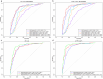
Results: We found statistically significant positive correlations between CSF and plasma Aβ42, Aβ42/Aβ40 ratio and pTau181. All the biomarkers, except Aβ40, showed differences in A+ vs. A-, A+T+ vs. A-T- and A+T- vs. A-T- patients. Moreover, Aβ42 and Aβ42/Aβ40 plasma levels were lower in A+T- compared to A-T- and A-T+ groups, and pTau181 and pTau217 plasma levels were higher in A+T+ compared to A+T-. Aβ42/Aβ40 and pTau217 showed a robust performance in distinguishing A+ from A- (AUC = 0.857 and 0.862, respectively) and A+T+ from A-T- (AUC = 0.866 and 0.911) subjects.
Conclusions: Our results suggest that plasma biomarkers, and especially Aβ42/Aβ40 ratio and pTau217, are promising candidates for the early detection of AD pathology.
Keywords: Alzheimer’s disease; Aβ; Biomarkers; CSF; Diagnosis; Lumipulse®; Mild Cognitive Impairment; Plasma; pTau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in compliance with the Declaration of Helsinki for the protection of human participants and was approved by the Ethics Committee of Fondazione Policlinico Universitario Agostino Gemelli (approval code 2251). Additionally, approval was obtained from all local ethics committees of the participating clinical centres. Written informed consent was obtained from participants. Consent for publication: N/A. Competing interests: A.P. is an employee of Fujirebio.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

