Machine learning models for dementia screening to classify brain amyloid positivity on positron emission tomography using blood markers and demographic characteristics: a retrospective observational study
- PMID: 39838434
- PMCID: PMC11748352
- DOI: 10.1186/s13195-024-01650-1
Machine learning models for dementia screening to classify brain amyloid positivity on positron emission tomography using blood markers and demographic characteristics: a retrospective observational study
Abstract
Background: Intracerebral amyloid β (Aβ) accumulation is considered the initial observable event in the pathological process of Alzheimer's disease (AD). Efficient screening for amyloid pathology is critical for identifying patients for early treatment. This study developed machine learning models to classify positron emission tomography (PET) Aβ-positivity in participants with preclinical and prodromal AD using data accessible to primary care physicians.
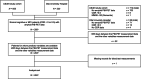
Methods: This retrospective observational study assessed the classification performance of combinations of demographic characteristics, routine blood test results, and cognitive test scores to classify PET Aβ-positivity using machine learning. Participants with mild cognitive impairment (MCI) or normal cognitive function who visited Oita University Hospital or had participated in the USUKI study and met the study eligibility criteria were included. The primary endpoint was assessment of the classification performance of the presence or absence of intracerebral Aβ accumulation using five machine learning models (i.e., five combinations of variables), each constructed with three classification algorithms, resulting in a total of 15 patterns. L2-regularized logistic regression, and kernel Support Vector Machine (SVM) and Elastic Net algorithms were used to construct the classification models using 34 pre-selected variables (12 demographic characteristics, 11 blood test results, 11 cognitive test results).
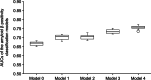
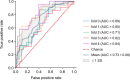
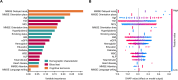
Results: Data from 262 records (260 unique participants) were analyzed. The mean (standard deviation [SD]) participant age was 73.8 (7.8) years. Using L2-regularized logistic regression, the mean receiver operating characteristic (ROC) area under the curve (AUC) (SD) in Model 0 (basic demographic characteristics) was 0.67 (0.01). Classification performance was similar in Model 1 (basic demographic characteristics and Mini Mental State Examination [MMSE] subscores) and Model 2 (demographic characteristics and blood test results) with a cross-validated mean ROC AUC (SD) of 0.70 (0.01) for both. Model 3 (demographic characteristics, blood test results, MMSE subscores) and Model 4 (Model 3 and ApoE4 phenotype) showed improved performance with a mean ROC AUC (SD) of 0.73 (0.01) and 0.76 (0.01), respectively. In models using blood test results, thyroid-stimulating hormone and mean corpuscular volume tended to be the largest contributors to classification. Classification performances were similar using the SVM and Elastic Net algorithms.
Conclusions: The machine learning models used in this study were useful for classifying PET Aβ-positivity using data from routine physician visits.
Trial registration: UMIN Clinical Trials Registry (UMIN000051776, registered on 31/08/2023).
Keywords: AD; Alzheimer’s disease; Amyloid β positivity; Dementia; Machine learning.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the local ethics committee of Oita University Hospital. Given the difficulty of obtaining informed consent for existing patient information, an information disclosure document (approved by the ethics committee and authorized by the head of Oita University Hospital) that included information on the conduct of this study and the study purpose was made publicly available on the university website. The study was registered with the University hospital Medical Information Network Clinical Trials Registry (UMIN000051776). Consent for publication: Not applicable. Competing interests: NK has received consultancy/speaker fees from Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Sumitomo Pharma Co., Ltd., PDRadiopharma Inc., Otsuka Pharmaceutical Co., Ltd., and Eli Lilly Japan K.K. KS, MM, MK and YN are employees of Eisai Co., Ltd. TM, TA, and EM have no competing interest to declare.
Figures
Similar articles
-
(11)C-PIB-PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jul 23;2014(7):CD010386. doi: 10.1002/14651858.CD010386.pub2. Cochrane Database Syst Rev. 2014. PMID: 25052054 Free PMC article.
-
¹⁸F-FDG PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD010632. doi: 10.1002/14651858.CD010632.pub2. Cochrane Database Syst Rev. 2015. PMID: 25629415 Free PMC article.
-
18F PET with flutemetamol for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012884. doi: 10.1002/14651858.CD012884. Cochrane Database Syst Rev. 2017. PMID: 29164602 Free PMC article.
-
Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. Cochrane Database Syst Rev. 2014. PMID: 24913723 Free PMC article.
-
Timeline to symptomatic Alzheimer's disease in people with Down syndrome as assessed by amyloid-PET and tau-PET: a longitudinal cohort study.Lancet Neurol. 2024 Dec;23(12):1214-1224. doi: 10.1016/S1474-4422(24)00426-5. Lancet Neurol. 2024. PMID: 39577922
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

