A comparative analysis in monitoring 24-hour urinary copper in wilson disease: sampling on or off treatment?
- PMID: 39838467
- PMCID: PMC11748325
- DOI: 10.1186/s13023-025-03545-2
A comparative analysis in monitoring 24-hour urinary copper in wilson disease: sampling on or off treatment?
Abstract
Background & aim: Twenty-four-hour urinary copper excretion (24 h-UCE) is the standard diagnostic tool for dose adjustments in maintenance therapy in Wilson disease (WD) patients. Guidelines lack data if both variants of 24 h-UCE measurement (with or without 48 h of treatment interruption) are equally interpretable.
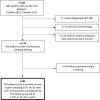
Methods: Eighty-four patients with a confirmed diagnosis of WD treated with chelators (50% of patients with D-Penicillamine and 50% with trientine) and with pairwise 24-h-UCE values on-therapy and off-therapy were included in the analysis. Pairwise urinary sampling between October 2022 (T0) and a 12-month FU (T2) was compared, and exchangeable copper (CuEXC) was additionally measured at T0.
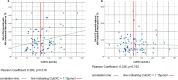
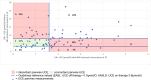
Results: Among the 84 patients, 65% had predominant hepatic symptoms, the median age was 42 years, and 58% were female. At T0, patients were in the stable maintenance phase, with a median treatment duration of 21.9 years. The levels of the biochemical markers liver and copper metabolism remained stable over the 12-month observation period for all patients. 24 h-UCE off-therapy significantly decreased from T0 to T2 (p = 0.03), whereas no statistically significant differences were detected for 24 h-UCE after therapy. Both sampling methods did not correlate. CuEXC was significantly correlated with 24 h-UCE after 48 h of dose interruption (p = 0.018) but not with 24 h-UCE after therapy. A total of 46% of the 24 h-UCE value pairs were discordant, laying out the aimed therapeutic ranges given in current international guidelines.
Conclusion: Off-therapy 24 h-UCE reflects the "free" copper pool more accurately than does urinary sampling. The study shows discordant results for both sampling methods in approximately half of the patients, revealing that interpretation of 24 h-UCE with respect to chelator-dosing decisions should be performed with caution.
Keywords: Exchangeable copper; Monitoring; Urinary copper; Wilson disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The retrospective data collection was approved by the Ethics Committee of the University of Heidelberg (protocol number: S-565/2011), which complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws and fulfilled Regulation (EU) 2016/679 of the European Parliament and the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data (ID number DSAN854-A-OS/5). Consent for publication: Written informed consent for the procedure and management was obtained from all individual participants included in the study. Competing interests: IM advises for Univar, received travel grants from Univar and received speaker fees from Orphalan. PL, CW, VYL, SK, AO, ML, AL, PD, MG, SW and HZ declare no conflicts of interest. PM received honoraria from Falk, Orphalan and AstraZeneca. AP has received research (institutional) grants from Orphalan, AddMedica and Alexion; consulting fees from Orphalan, Univar, Alexion and Vivet Therapeutics; and speaker fees from Alexion and Orphalan. KHW has received research (institutional) grants from Orphalan; consulting fees from Orphalan, Univar, Pfizer, Alexion and Vivet Therapeutics; speaker fees from Falk, AbbVie, Alexion, and Orphalan; travel assistance to meetings from Alexion and Univar; and advisory board payments from Ultragenyx. UM received honoraria for teaching from Falk and Univar and received travel grants from Gilead and Falk.
Figures

References
-
- Walshe JM. Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet. 1982;1(8273):643–7. - PubMed
-
- European Association for Study of L. EASL Clinical Practice guidelines: Wilson’s disease. J Hepatol. 2012;56(3):671–85. - PubMed
-
- Walshe JM. Wilson’s disease; new oral therapy. Lancet. 1956;270(6906):25–6. - PubMed
-
- Walshe JM. Penicillamine, a new oral therapy for Wilson’s disease. Am J Med. 1956;21(4):487–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

