Succinate receptor 1 signaling mutually depends on subcellular localization and cellular metabolism
- PMID: 39838520
- PMCID: PMC12001207
- DOI: 10.1111/febs.17407
Succinate receptor 1 signaling mutually depends on subcellular localization and cellular metabolism
Abstract
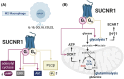
Succinate is a pivotal tricarboxylic acid cycle metabolite but also specifically activates the Gi- and Gq-coupled succinate receptor 1 (SUCNR1). Contradictory roles of succinate and succinate-SUCNR1 signaling include reports about its anti- or pro-inflammatory effects. The link between cellular metabolism and localization-dependent SUCNR1 signaling qualifies as a potential cause for the reported conflicts. To systematically address this connection, we used a diverse set of methods, including several bioluminescence resonance energy transfer-based biosensors, dynamic mass redistribution measurements, second messenger and kinase phosphorylation assays, calcium imaging, and metabolic analyses. Different cellular metabolic states were mimicked using glucose (Glc) or glutamine (Gln) as available energy substrates to provoke differential endogenous succinate (SUC) production. We show that SUCNR1 signaling, localization, and metabolism are mutually dependent, with SUCNR1 showing distinct spatial and energy substrate-dependent Gi and Gq protein activation. We found that Gln-consumption associated with a higher rate of oxidative phosphorylation causes increased extracellular SUC concentrations, accompanied by a higher rate of SUCNR1 internalization, reduced miniGq protein recruitment to the plasma membrane, and lower Ca2+ signals. In Glc, under basal conditions, SUCNR1 causes stronger Gq than Gi protein activation, while the opposite is true upon stimulation with an agonist. In addition, SUCNR1 specifically interacts with miniG proteins in endosomal compartments. In THP-1 cells, polarized to M2-like macrophages, endogenous SUCNR1-mediated Gi signaling stimulates glycolysis, while Gq signaling inhibits the glycolytic rate. Our results suggest that the metabolic context determines spatially dependent SUCNR1 signaling, which in turn modulates cellular energy homeostasis and mediates adaptations to changes in SUC concentrations.
Keywords: SUCNR1; macrophages; metabolism; signal transduction; succinate.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling.Cell Rep. 2021 Jun 15;35(11):109246. doi: 10.1016/j.celrep.2021.109246. Cell Rep. 2021. PMID: 34133934
-
Cell selectivity in succinate receptor SUCNR1/GPR91 signaling in skeletal muscle.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E289-E298. doi: 10.1152/ajpendo.00009.2023. Epub 2023 Feb 22. Am J Physiol Endocrinol Metab. 2023. PMID: 36812387
-
Succinate receptor 1 inhibits mitochondrial respiration in cancer cells addicted to glutamine.Cancer Lett. 2022 Feb 1;526:91-102. doi: 10.1016/j.canlet.2021.11.024. Epub 2021 Nov 20. Cancer Lett. 2022. PMID: 34813893
-
Rethinking succinate: an unexpected hormone-like metabolite in energy homeostasis.Trends Endocrinol Metab. 2021 Sep;32(9):680-692. doi: 10.1016/j.tem.2021.06.003. Epub 2021 Jul 20. Trends Endocrinol Metab. 2021. PMID: 34301438 Review.
-
Succinate: a metabolic signal in inflammation.Trends Cell Biol. 2014 May;24(5):313-20. doi: 10.1016/j.tcb.2013.11.008. Epub 2013 Dec 19. Trends Cell Biol. 2014. PMID: 24361092 Review.
References
-
- Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y & Tones MA (2007) Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens 20, 1209–1215. - PubMed
-
- Hochachka PW & Dressendorfer RH (1976) Succinate accumulation in man during exercise. Eur J Appl Physiol Occup Physiol 35, 235–242. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

