Update on the treatment navigation for functional cure of chronic hepatitis B: Expert consensus 2.0
- PMID: 39838828
- PMCID: PMC11925436
- DOI: 10.3350/cmh.2024.0780
Update on the treatment navigation for functional cure of chronic hepatitis B: Expert consensus 2.0
Abstract
As new evidence emerges, treatment strategies toward the functional cure of chronic hepatitis B are evolving. In 2019, a panel of national hepatologists published a Consensus Statement on the functional cure of chronic hepatitis B. Currently, an international group of hepatologists has been assembled to evaluate research since the publication of the original consensus, and to collaboratively develop the updated statements. The 2.0 Consensus was aimed to update the original consensus with the latest available studies, and provide a comprehensive overview of the current relevant scientific literatures regarding functional cure of hepatitis B, with a particular focus on issues that are not yet fully clarified. These cover the definition of functional cure of hepatitis B, its mechanisms and barriers, the effective strategies and treatment roadmap to achieve this endpoint, in particular new surrogate biomarkers used to measure efficacy or to predict response, and the appropriate approach to pursuing a functional cure in special populations, the development of emerging antivirals and immunomodulators with potential for curing hepatitis B. The statements are primarily intended to offer international guidance for clinicians in their practice to enhance the functional cure rate of chronic hepatitis B.
Keywords: Antiviral Agents; Chronic hepatitis B; Consensus; HBsAg; Immunologic Factors.
Conflict of interest statement
DW: Nothing to disclose. JHK: Nothing to disclose. TP: Research grants: Roche Diagnostic, Janssen, VIR, GSK, Sysmex; Honoraria: Roche, Takeda, DKSH, Viatris, Eisai, Astra Zeneca, Sysmex and American Taiwan Biopharm. XJW: Nothing to disclose. PTFK: Research grants: VIR Biotechnology Inc and Gilead Sciences; Consulting fees: Abbott Diagnostics, Aligos, BlueJay, Gilead Sciences, GSK, and Assembly Biosciences; Honoraria: Gilead Sciences and GSK. MO: Nothing to disclose. SHA: Nothing to disclose. YT: Research grants: Gilead Sciences, FUJIREBIO Inc, Sysmex Corp, Janssen Pharmaceutical K.K. and GSK; Honoraria: Gilead Sciences, GSK, and HU frontier. GQW: Nothing to disclose. ZHY: Nothing to disclose.
WHL: Nothing to disclose. YSL: Research grants, consulting fees and honoraria: Gilead Sciences. JQN: Nothing to disclose. FML: Nothing to disclose. WHZ: Nothing to disclose. ZLG: Nothing to disclose. AK: Research grants: Roche, Roche Diagnostics, and Abbott Laboratories; Honoraria: Roche, Roche Diagnostics, Abbott Laboratories, and Esai. MFH: Nothing to disclose. WMY: Nothing to disclose. HR: Nothing to disclose. PH: Nothing to disclose. SNS: Nothing to disclose. PYK: Research grants: Altimmune, Arrowhead, Gilead, Novo Nordisk, Target Registries, Ultragenyx, Salix, Madrigal, Ausper Bio, Takeda; Consulting fees: Abbvie, Aligos, Ausper Bio, Durect, Generon, Genentch, Gilead, Drug Farm, HepQuant, Inventiva, Mallinckrodt, Mirum, NovoNordisk, Surrozen. FSW: Nothing to disclose. MFY: Research grants: AbbVie, Assembly Biosciences, Arrowhead Pharmaceuticals, Fujirebio Incorporation, Gilead Sciences, Immunocore, Sysmex Corporation and Roche; Consulting fees: AbbVie, Abbott Diagnotics, Aligos Therapeutics, AiCuris, Antios Therapeutics, Arbutus Biopharma, Arrowhead Pharmaceuticals, Assembly Biosciences, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GlaxoSmith-Kline, Gilead Sciences, Immunocore, Janssen, Precision BioSciences, Roche, Sysmex Corporation, Tune Therapeutics, Vir Biotechnology and Visirna Therapeutics; Honoraria: Fujirebio Incorporation, Gilead Sciences, Roche, Sysmex Corporation. QN: Research grants: MSD, Roche, NOVARTIS, BMS, Gilead Sciences and GSK; Consulting fees: MSD, Roche, NOVARTIS, BMS, Gilead Sciences and GSK.
Figures
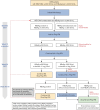

References
-
- WHO. Hepatitis B. WHO web site, < https://www.who.int/news-room/fact-sheets/detail/hepatitis-b> Accessed 1 Jan 2025.
-
- Ning Q, Wu D, Wang GQ, Ren H, Gao ZL, Hu P, et al. Roadmap to functional cure of chronic hepatitis B: An expert consensus. J Viral Hepat. 2019;26:1146–1155. - PubMed
-
- Yip TC, Wong GL, Chan HL, Tse YK, Lam KL, Lui GC, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. 2019;70:361–370. - PubMed
-
- Kim MA, Kim SU, Sinn DH, Jang JW, Lim YS, Ahn SH, et al. Discontinuation of nucleos(t)ide analogues is not associated with a higher risk of HBsAg seroreversion after antiviral-induced HBsAg seroclearance: a nationwide multicentre study. Gut. 2020;69:2214–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

