The relationship between spontaneous cystic degeneration and pseudocapillarization in sinusoids in the liver of aged Sprague-Dawley rats
- PMID: 39839718
- PMCID: PMC11745498
- DOI: 10.1293/tox.2024-0034
The relationship between spontaneous cystic degeneration and pseudocapillarization in sinusoids in the liver of aged Sprague-Dawley rats
Abstract
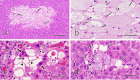
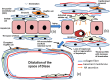
Cystic degeneration (CD) in the liver is a cyst-like lesion composed of one or more pseudocysts lacking lining cells, occurring spontaneously in rats older than 12 months, with a male predilection. In this study, 32 CDs were identified in 23 out of 104 non-treated, control male Sprague-Dawley rats from two combined chronic toxicity and carcinogenicity studies with agrochemicals. They were examined histologically, histochemically, and immunohistochemically to assess the pathogenesis and pathological significance of CD, focusing on pseudocapillarization in aged rat liver. Pseudocapillarization refers to age-related capillarization of hepatic sinusoids and is distinct from sinusoidal capillarization observed in hepatic cirrhosis. Both CD and pseudocapillarization, characterized by factor VIII-related antigen expression, were primarily noted in the periportal regions of the rat liver. CD areas exhibited enhanced vimentin expression in a diffuse linear pattern in their septa with occasional focal linear α-smooth muscle actin expression and the fluid containing hyaluronic acid accumulated in their lumen that are thought to be formed by hepatocellular apoptosis. These findings suggest a series of reactive changes associated with hepatocellular apoptosis due to pseudocapillarization in the sinusoids. In conclusion, spontaneous CD in rat liver is not a degenerative lesion or cystic enlargement of stellate cells, but a structural abnormality in pre-existing liver tissue resulting from aging-related changes in sinusoidal endothelial cells and hepatocytes. Pseudocapillarization of sinusoids is considered a precursor lesion of CD in the rat liver.
Keywords: aging; cystic degeneration; liver; pseudocapillarization; rat; spongiosis hepatis.
©2025 The Japanese Society of Toxicologic Pathology.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Figures


Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Curcumin ameliorates intrahepatic angiogenesis and capillarization of the sinusoids in carbon tetrachloride-induced rat liver fibrosis.Toxicol Lett. 2013 Sep 12;222(1):72-82. doi: 10.1016/j.toxlet.2013.06.240. Epub 2013 Jul 8. Toxicol Lett. 2013. PMID: 23845850
-
Studies on capillarization of the hepatic sinusoids in alcoholic liver disease.Alcohol Alcohol Suppl. 1993;1B:77-84. doi: 10.1093/alcalc/28.supplement_1b.77. Alcohol Alcohol Suppl. 1993. PMID: 7516164
-
Angiopoietin/tie receptors system may play a role during reconstruction and capillarization of the hepatic sinusoids after partial hepatectomy and liver necrosis in rats.Hepatol Res. 2004 May;29(1):51-59. doi: 10.1016/j.hepres.2004.02.004. Hepatol Res. 2004. PMID: 15135347
-
Alcohol-associated capillarization of sinusoids: A critique since the discovery by Schaffner and Popper in 1963.Anat Rec (Hoboken). 2022 Jul;305(7):1592-1610. doi: 10.1002/ar.24829. Epub 2021 Nov 23. Anat Rec (Hoboken). 2022. PMID: 34766732 Review.
References
-
- Foster JR. Liver. In: Boorman’s Pathology of the Rat. Reference and Atlas, 2nd ed. AW Suttie. (ed). Academic Press, Cambridge. 81–105. 2018.
-
- Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, and Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(Suppl): 5S–81S. 2010. - PubMed
-
- Bannasch P, Bloch M, and Zerban H. Spongiosis hepatis. Specific changes of the perisinusoidal liver cells induced in rats by N-nitrosomorpholine. Lab Invest. 44: 252–264. 1981. - PubMed
-
- Bannasch P, Zerban H, and Fugel H-J. Spongiosis hepatis, rat. In: Digestive System. Monographs on Pathology of Laboratory Animals Sponsored by the International Life Sciences Institute. TC Jones, U Mohr, RD Hunt (eds). Springer-Verlag, Berlin. 116–123. 1985.
-
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. In: GH Bourne, JF Danielli, and KW Jeon (eds). Int Rev Cytol. Acadenic Press, Cambridge. 66: 303–353. 1980. - PubMed

