Granulomatous inflammatory responses are elicited in the liver of PD-1 knockout mice by de novo genome mutagenesis
- PMID: 39839810
- PMCID: PMC11744370
- DOI: 10.1093/discim/kyae018
Granulomatous inflammatory responses are elicited in the liver of PD-1 knockout mice by de novo genome mutagenesis
Erratum in
-
Correction to: Granulomatous inflammatory responses are elicited in the liver of PD-1 knockout mice by de novo genome mutagenesis.Discov Immunol. 2025 Jun 5;4(1):kyaf010. doi: 10.1093/discim/kyaf010. eCollection 2025. Discov Immunol. 2025. PMID: 40475177 Free PMC article.
Abstract
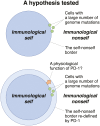
Introduction: Programmed death-1 (PD-1) is a negative regulator of immune responses. Upon deletion of PD-1 in mice, symptoms of autoimmunity developed only after they got old. In a model experiment in cancer immunotherapy, PD-1 was shown to prevent cytotoxic T lymphocytes from attacking cancer cells that expressed neoantigens derived from genome mutations. Furthermore, the larger number of genome mutations in cancer cells led to more robust anti-tumor immune responses after the PD-1 blockade. To understand the common molecular mechanisms underlying these findings, we hypothesize that we might have acquired PD-1 during evolution to avoid/suppress autoimmune reactions against neoantigens derived from mutations in the genome of aged individuals.
Methods: To test the hypothesis, we introduced random mutations into the genome of young PD-1-/- and PD-1+/+ mice. We employed two different procedures of random mutagenesis: administration of a potent chemical mutagen N-ethyl-N-nitrosourea (ENU) into the peritoneal cavity of mice and deletion of MSH2, which is essential for the mismatch-repair activity in the nucleus and therefore for the suppression of accumulation of random mutations in the genome.
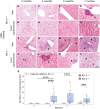
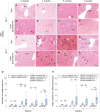
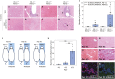
Results: We observed granulomatous inflammatory changes in the liver of the ENU-treated PD-1 knockout (KO) mice but not in the wild-type (WT) counterparts. Such lesions also developed in the PD-1/MSH2 double KO mice but not in the MSH2 single KO mice.
Conclusion: These results support our hypothesis about the physiological function of PD-1 and address the mechanistic reasons for immune-related adverse events observed in cancer patients having PD-1-blockade immunotherapies.
Keywords: PD-1; autoimmunity; genome mutation; granuloma; self-nonself discrimination.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
Y.I. received a research grant from ONO Pharmaceutical Co., LTD. (Osaka, Japan). The other authors declare no conflicts of interest.
Figures



Similar articles
-
B Cells Are Required to Generate Optimal Anti-Melanoma Immunity in Response to Checkpoint Blockade.Front Immunol. 2022 May 26;13:794684. doi: 10.3389/fimmu.2022.794684. eCollection 2022. Front Immunol. 2022. PMID: 35720386 Free PMC article.
-
Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses.Proc Natl Acad Sci U S A. 2015 May 26;112(21):6682-7. doi: 10.1073/pnas.1420370112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964334 Free PMC article.
-
DNA mismatch repair deficiency stimulates N-ethyl-N-nitrosourea-induced mutagenesis and lymphomagenesis.Cancer Res. 2003 May 1;63(9):2062-6. Cancer Res. 2003. PMID: 12727820
-
Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy.J Hematol Oncol. 2019 May 31;12(1):54. doi: 10.1186/s13045-019-0738-1. J Hematol Oncol. 2019. PMID: 31151482 Free PMC article. Review.
-
Why is immunotherapy effective (or not) in patients with MSI/MMRD tumors?Bull Cancer. 2019 Feb;106(2):105-113. doi: 10.1016/j.bulcan.2018.08.007. Epub 2018 Oct 17. Bull Cancer. 2019. PMID: 30342749 Review.
References
-
- Ishida Y, Agata Y, Shibahara K, Honjo T.. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992, 11, 3887–95. doi: https://doi.org/ 10.1002/j.1460-2075.1992.tb05481.x - DOI - PMC - PubMed
-
- Okazaki T, Honjo T.. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007, 19, 813–24. doi: https://doi.org/ 10.1093/intimm/dxm057 - DOI - PubMed
-
- Sharpe AH, Pauken KE.. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018, 18, 153–67. doi: https://doi.org/ 10.1038/nri.2017.108 - DOI - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T.. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–51. doi: https://doi.org/ 10.1016/s1074-7613(00)80089-8 - DOI - PubMed
-
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–22. doi: https://doi.org/ 10.1126/science.291.5502.319 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
