A bioluminescent reporter bioassay for in-process assessment of chimeric antigen receptor lentiviral vector potency
- PMID: 39839908
- PMCID: PMC11744306
- DOI: 10.1093/abt/tbae032
A bioluminescent reporter bioassay for in-process assessment of chimeric antigen receptor lentiviral vector potency
Abstract
Background: Chimeric antigen receptor (CAR)-T-cell therapy is a breakthrough in the field of cancer immunotherapy, wherein T cells are genetically modified to recognize and attack cancer cells. Delivery of the CAR gene is a critical step in this therapy and is usually achieved by transducing patient T cells with a lentiviral vector (LV). Because the LV is an essential component of CAR-T manufacturing, there is a need for simple bioassays that reflect the mechanism of action (MOA) of the LV and can measure LV potency with accuracy and specificity. Common methods for LV quantification may overestimate functional titer and lack a functional readout of LV MOA.
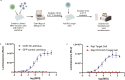
Methods: We developed a bioluminescent reporter bioassay using Jurkat T cells stably expressing a luciferase reporter under the control of an nuclear factor of activated T cells (NFAT) response element and tested its suitability for measuring LV potency.
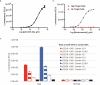
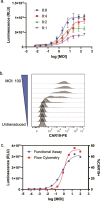
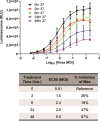
Results: Jurkat reporter cells can be transduced with CAR LV and combined with target cells, yielding a luminescent signal that is dependent on the identity and potency of the LV used. Bioluminescence was highly correlated with CAR expression. The assay is stability indicating and suitable for use in drug development and quality control settings.
Conclusions: We have developed a simple bioassay for potency testing of CAR LV. The bioassay represents a significant improvement over other approaches to LV quantification because it reflects the MOA of the LV and selectively detects fully functional viral particles, making it ideal for inclusion in a matrix of in-process quality control assays for CAR LV.
Keywords: CAR-T; bioassay; lentivirus; potency assay.
© The Author(s) 2024. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
The authors are employees of Promega Corp.
Figures
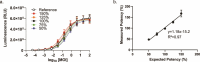
References
-
- Shirasu N, Shibaguci H, Kuroki M. et al. Construction and molecular characterization of human chimeric T-cell antigen receptors specific for carcinoembryonic antigen. Anticancer Res 2010;30:2731–8. - PubMed
LinkOut - more resources
Full Text Sources
