Humanized anti-CD11d monoclonal antibodies suitable for basic research and therapeutic applications
- PMID: 39839909
- PMCID: PMC11744312
- DOI: 10.1093/abt/tbae031
Humanized anti-CD11d monoclonal antibodies suitable for basic research and therapeutic applications
Abstract
Background: Immunomodulatory agents targeting the CD11d/CD18 integrin are in development for the treatment of several pathophysiologies including neurotrauma, sepsis, and atherosclerosis. Murine anti-human CD11d therapeutic antibodies have successfully improved neurological and behavioral recovery in rodent neurotrauma models. Here, we present the progression of CD11d-targeted agents with the development of humanized anti-CD11d monoclonal antibodies.
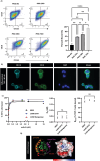
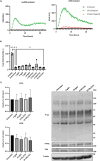
Methods: Primary human leukocytes and the THP-1 monocytic cell line were used to determine the binding of the CD11d antibodies, determine binding affinities, and assess outside-in signaling induced by CD11d antibody binding. In addition, a rat model of spinal cord injury was employed to demonstrate that the humanized monoclonal antibodies retained their therapeutic function in vivo. These determinations were made using a combination of flow cytometry, western blotting, immunohistochemistry, biochemical assays, and a locomotor behavioral assessment.
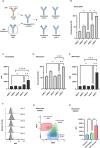
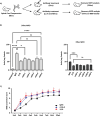
Results: Flow cytometric analysis demonstrated that the humanized anti-CD11d clones bind both human monocytes and neutrophils. Using a THP-1 model, the humanized anti-CD11d-2 clone was then determined to bind both the active and inactive CD11d/CD18 conformations without inducing inflammatory cell signaling. Finally, an investigation using anti-CD11d-2 as a detection tool uncovered a mismatch between total and surface-level CD11d and CD18 expression that was not altered by CK2 inhibition.
Conclusions: By developing humanized anti-CD11d monoclonal antibodies, new tools are now available to study CD11d biology and potentially treat inflammation arising from acute neurotrauma via CD11d targeting.
Keywords: CD11dCD18; integrin; monoclonal antibody; therapeutic antibody.
© The Author(s) 2024. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
The authors share the following patents with Eli Lilly & Company:
Figures

Similar articles
-
A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats.J Neurotrauma. 2012 Sep 20;29(14):2375-92. doi: 10.1089/neu.2012.2408. Epub 2012 Jul 12. J Neurotrauma. 2012. PMID: 22676851 Free PMC article.
-
Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats.J Neurochem. 2005 Sep;94(5):1361-73. doi: 10.1111/j.1471-4159.2005.03280.x. Epub 2005 Jun 30. J Neurochem. 2005. PMID: 15992367
-
CD11d Antibody Treatment Improves Recovery in Spinal Cord-Injured Mice.J Neurotrauma. 2012 Feb 10;29(3):539-50. doi: 10.1089/neu.2011.1976. Epub 2011 Dec 20. J Neurotrauma. 2012. PMID: 22044160 Free PMC article.
-
The effectiveness of the anti-CD11d treatment is reduced in rat models of spinal cord injury that produce significant levels of intraspinal hemorrhage.Exp Neurol. 2017 Sep;295:125-134. doi: 10.1016/j.expneurol.2017.06.002. Epub 2017 Jun 3. Exp Neurol. 2017. PMID: 28587875
-
β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration.Front Immunol. 2021 Nov 8;12:775447. doi: 10.3389/fimmu.2021.775447. eCollection 2021. Front Immunol. 2021. PMID: 34858434 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
