Discovery of a common light chain bispecific antibody targeting PD-1 and PD-L1 by Hybridoma-to-Phage-to-Yeast (H2PtY) platform
- PMID: 39839911
- PMCID: PMC11744305
- DOI: 10.1093/abt/tbae027
Discovery of a common light chain bispecific antibody targeting PD-1 and PD-L1 by Hybridoma-to-Phage-to-Yeast (H2PtY) platform
Abstract
Background: Therapeutic antibody drugs targeting the PD-1 pathway are generally characterized by relatively low response rates and susceptibility to drug resistance during clinical application. Therefore, there is an urgent need for alternative therapeutic strategies to increase the immune response rate. Bispecific antibodies co-targeting PD-1 and PD-L1 may have greater potential to improve the efficacy of the immune checkpoint pathway.
Method: In this study, we developed a potent humanized common light chain (CLC) IgG shape bispecific antibody (bsAb), named JMB2005, based on Hybridoma-to-Phage-to-Yeast platform. The platform allowed us to discover CLC bsAb from traditional mice for any pair of given targets.
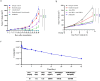
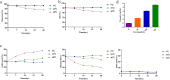
Results: JMB2005 exhibited favorable developability, good manufacturing property, and satisfactory efficacy, which could be given via subcutaneous injection at the concentration of 120 mg/mL. Mechanistically, JMB2005 could bridge tumor cells and T cells with both Fab arms and promote T-cells to function as direct tumor cell killers. It could also promote T cell activation by blocking the binding of PD-L1 to CD80. Furthermore, JMB2005 has exhibited a favorable half-life and has demonstrated promising anti-tumor therapeutic efficacy in vivo.
Conclusion: Consequently, the present study showed that the novel humanized CLC bsAb JMB2005 may represent a novel therapeutic agent of great clinical potential.
Keywords: JMB2005; PD-1; PD-L1; bispecific antibody; common light chain.
© The Author(s) 2024. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
Peipei Liu, Chunyin Gu, Xiaodan Cao, Huawei Zhang, Zongda Wang, Yukun Yang, KeDong OuYang, Yingying Zhen, Fangfang Jia, Xianqing He, Haixiang Yu, and Sujun Deng are employees of Shanghai Jemincare Pharmaceutical Co., Ltd.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
