Impact of Organ Donor Pretreatment With Anti-Thymocyte Globulin in a Murine Model of Allogenic Kidney Transplantation
- PMID: 39839912
- PMCID: PMC11745874
- DOI: 10.3389/ti.2024.13997
Impact of Organ Donor Pretreatment With Anti-Thymocyte Globulin in a Murine Model of Allogenic Kidney Transplantation
Abstract
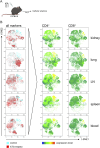
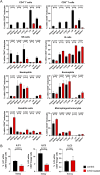
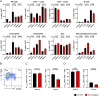
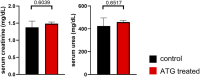
Kidney transplantation is the treatment of choice for end-stage organ failure. To improve transplantation outcomes, particularly of "marginal" organs from extended criteria donors (ECD), attempts have been made to therapeutically modulate donor or graft pre-transplantation. Anti-thymocyte globulin (ATG) has a history as lymphocyte-depleting, immunosuppressive drug for treating rejection episodes post transplantation. In this study, however, we aimed to comprehensively analyze the effects of ATG donor pre-conditioning in a mouse model of kidney transplantation. ATG pre-treatment of potential donors led to a broad depletion of T- and NK cells in peripheral blood, non-lymphoid (including kidney) and lymphoid organs within 48 h, whereas myeloid cells were spared. ATG was also effectively depleting renal innate lymphoid type 1 and 2 cells. Importantly, transplantation of kidneys from ATG pre-treated donors into fully mismatched recipients showed only mild effects on leukocyte re-composition post transplantation. In line with this, serum creatinine and urea levels were similar in animals receiving kidneys from ATG treated donors or controls, demonstrating that donor treatment had no effect on allograft function in the early post-transplantation phase. In summary, our findings are suggestive of a more cell-type-specific depletion strategy in concert with an experimental model better reflecting aspects of clinical transplantation.
Keywords: anti-thymocyte globulin; donor pre-treatment; kidney function; kidney transplantation; passenger leukocytes.
Copyright © 2025 He, Yang, Kotsch and Sattler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mazeti-Felicio CM, Caldas HC, Fernandes-Charpiot IMM, Dezotti CZ, Baptista M, Abbud-Filho M. Preimplantation Kidney Biopsies of Extended Criteria Donors Have a Heavier Inflammatory Burden Than Kidneys From Standard Criteria Donors. Transpl Direct (2017) 3:e180. 10.1097/TXD.0000000000000671 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

