Palmitoylethanolamide supplementation for human health: A state-of-the-art systematic review of Randomized Controlled Trials in patient populations
- PMID: 39839988
- PMCID: PMC11745966
- DOI: 10.1016/j.bbih.2024.100927
Palmitoylethanolamide supplementation for human health: A state-of-the-art systematic review of Randomized Controlled Trials in patient populations
Abstract
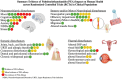
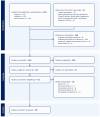
Interest in preventative dietary interventions for human health has increasingly focused on the endocannabinoid (eCB)-like compound palmitoylethanolamide (PEA), a bioactive lipid mediator with anti-inflammatory, analgesic, and neuroprotective properties. This Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020-compliant systematic review aimed at collecting and comprehensively discussing all available data from Randomized Controlled Trials (RCTs) evaluating the efficacy and tolerability of PEA supplementation across human illnesses in patient populations. Overall, 48 eligible outputs from 47 RCTs were extracted, covering neuropsychiatric (n = 15), neurological (n = 17), somatic (n = 13), and visceral (n = 11) disturbances, as well as PEA effects on blood/plasma or other tissue biomarkers (n = 10). The strongest evidence emerged from RCTs exploring PEA impact on pain management and measures of general wellbeing, especially in its ultramicronized/micronized or cold-water dispersible formulations, showing good tolerability compared to controls. Also, alongside symptom improvement, PEA demonstrated to modulate biomarkers early altered in the initial phases of an illness or contributing to its progression, suggesting a disease-modifying potential. This systematic review provided a comprehensive overview of the therapeutic potential of PEA across RCTs, highlighting its versatility either as monotherapy or add-on treatment for various clinical conditions.
Keywords: Cannabidiol; Endocannabinoidome; Glutamate; Neuroinflammation; Nutraceutical; Peroxisome proliferator-activated receptor α.
© 2024 The Authors.
Conflict of interest statement
MC has been a consultant/advisor to GW Pharma Limited, F. Hoffmann-La Roche Limited, and GW Pharma Italy SRL, outside of this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abedini T., Hosseyni R., Ghannadi F., Moghaddam H.S., Ardakani M.K., Talaei A., et al. Efficacy and safety of palmitoylethanolamide as an adjunctive treatment for acute mania: a randomized, double-blind, placebo-controlled trial. Psychiatr. Clin. Neurosci. 2022;76(10):505–511. - PubMed
-
- Andresen S.R., Bing J., Hansen R.M., Biering-Sorensen F., Johannesen I.L., Hagen E.M., et al. Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: a randomized, double-blind, placebo-controlled trial. Pain. 2016;157(9):2097–2103. - PubMed
-
- Arteaga-Henriquez G., Lugo-Marin J., Gisbert L., Setien-Ramos I., Martinez-Gallo M., Pujol-Borrell R., et al. Activation of the monocyte/macrophage system and abnormal blood levels of lymphocyte subpopulations in individuals with autism spectrum disorder: a systematic review and meta-analysis. Int. J. Mol. Sci. 2022;23(22) - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources