Eye reactions under the influence of drugs of abuse as measured by smartphones: a controlled clinical study in healthy volunteers
- PMID: 39840007
- PMCID: PMC11748063
- DOI: 10.3389/fnins.2024.1492246
Eye reactions under the influence of drugs of abuse as measured by smartphones: a controlled clinical study in healthy volunteers
Abstract
Background: It is known that illicit and prescribed drugs impact pupil size, eye movement and function. Still, comprehensive quantitative evaluations under known ambient light conditions are lacking, when smartphones are used for monitoring.
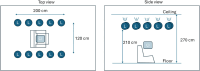
Methods: In this clinical study (NCT05731999), four medicinal products with addiction risks were administered to 48 subjects (18-70 years old, all with informed consent, 12 subjects per drug). Videos captured by smartphones at ~50 lux and ~ 500 lux documented the eye's reaction before and after controlled intake of single doses of oral oxycodone (20 mg), lorazepam (2 mg), lisdexamphetamine (70 mg) and inhaled cannabis flos (65 mg with 22% THC) over a 5-h test period. Data from three observational tests, non-convergence (NC, ability to cross the eyes), nystagmus (NY), and pupillary light reflex (PLR) were converted into 24 key features that represent different eye characteristics.
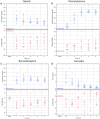
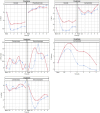
Results: Of the acquired data, 87-97% produced key features. At peak drug plasma concentration, oxycodone constricted pupils (p < 0.001); lorazepam induced non-convergence (p < 0.001); lisdexamphetamine induced dilated pupils (p < 0.001), irrespective of ambient light conditions. Inhaled cannabis induced miosis (p = 0.05 at ~50 lux, p = 0.10 at ~500 lux), a reduced light-induced amplitude (p = 0.003 at ~50 lux, p = 0.3 at ~500 lux) and redness of the sclerae (p = 0.14 at ~50 lux, p = 0.007 at ~500 lux). The drug effect lasted at least 5 h (p < 0.005) except for inhaled cannabis (2-3 h, p < 0.05).
Conclusion: The ocular response to oxycodone, lorazepam, lisdexamphetamine and cannabis, as measured under controlled light conditions using a smartphone-based assessment, demonstrated distinct and readily distinguishable patterns for each substance.
Clinical trial registration: Identifier, NTC05731999.
Keywords: benzodiazepines; cannabis; central stimulants; opioids; pupillometry; substance use disorder.
Copyright © 2025 Kuijpers, Andersson, Winkvist, Niesters, van Velzen, Nyberg, Dahan and Hämäläinen.
Conflict of interest statement
MW and MH are employees of Kontigo Care AB that have made a product partly based on the results of the presented results (Previct Drugs). KA is an employee of Skillsta Teknik Design och Kvalitet AB, which is a subcontractor to Kontigo Care AB. KA and MH are co-inventors of two (MH) and three (KA) different patents that are related to the presented work (PCT/SE2023/050638 “Quality assurance in body images”, PCT/SE2023/051070; “Method for estimating pupil size” (only KA), PCT/SE2023/051071; “Method and system for self-administered surveillance of use of addictive stimulus”). FN is part of the advisory board of Kontigo Care AB. AD reports receiving personal fees from Trevena and Enalare outside the submitted work and awards/grants from the US Food and Drug Administration and from the Dutch Research Council (NWO, the Hague, the Netherlands). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Kontigo Care AB. The funder had the following involvement in the study: Study design, interpretation of data, decision to publish and preparation of the manuscript. The funder was not involved in collection and analysis of data. The study was conducted in compliance with ISO 14155 to ensure that the study produced accurate data and reported unbiased findings. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Detecting benzodiazepine use through induced eye convergence inability with a smartphone app: a proof-of-concept study.Front Digit Health. 2025 May 30;7:1584716. doi: 10.3389/fdgth.2025.1584716. eCollection 2025. Front Digit Health. 2025. PMID: 40520217 Free PMC article.
-
Smartphone-based drug testing in the hands of patients with substance-use disorder-a usability study.Front Digit Health. 2024 Sep 2;6:1394322. doi: 10.3389/fdgth.2024.1394322. eCollection 2024. Front Digit Health. 2024. PMID: 39286832 Free PMC article.
-
Inhaled Δ9-tetrahydrocannabinol does not enhance oxycodone-induced respiratory depression: randomised controlled trial in healthy volunteers.Br J Anaesth. 2023 Apr;130(4):485-493. doi: 10.1016/j.bja.2022.12.018. Epub 2023 Jan 31. Br J Anaesth. 2023. PMID: 36725378 Clinical Trial.
-
Illicit drugs: Effects on eye.Indian J Med Res. 2019 Sep;150(3):228-238. doi: 10.4103/ijmr.IJMR_1210_17. Indian J Med Res. 2019. PMID: 31719293 Free PMC article. Review.
-
Cannabis-based medicines--GW pharmaceuticals: high CBD, high THC, medicinal cannabis--GW pharmaceuticals, THC:CBD.Drugs R D. 2003;4(5):306-9. doi: 10.2165/00126839-200304050-00005. Drugs R D. 2003. PMID: 12952500 Review.
Cited by
-
Effects of intravenous d9-THC on pupillary reaction and pupil size: a prospective, placebo-controlled trial in healthy volunteers not regularly consuming cannabis.BMC Ophthalmol. 2025 May 13;25(1):286. doi: 10.1186/s12886-025-04107-7. BMC Ophthalmol. 2025. PMID: 40355824 Free PMC article.
-
Correlation between orbital imaging features of thyroid-associated ophthalmopathy and pupillary light reflex measurement.Front Med (Lausanne). 2025 Mar 21;12:1552729. doi: 10.3389/fmed.2025.1552729. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40190576 Free PMC article.
-
Detecting benzodiazepine use through induced eye convergence inability with a smartphone app: a proof-of-concept study.Front Digit Health. 2025 May 30;7:1584716. doi: 10.3389/fdgth.2025.1584716. eCollection 2025. Front Digit Health. 2025. PMID: 40520217 Free PMC article.
References
-
- American Psychiatric Association DSM-5 Task Force (2013). Diagnostic and statistical manual of mental disorders: DSM-5™. 5th Edn. Washington, DC, USA American Psychiatric Publishing, Inc.
-
- Andersson K., Hämäläinen M., Zetterström A.. (2023). Quality assurance in medical facial images with respect to ambient illumination. WO2023249547A1. Available at: https://worldwide.espacenet.com/patent/search/family/089380360/publicati...
-
- Bech A. B., Clausen T., Waal H., Vindenes V., Edvardsen H. E., Frost J., et al. . (2020). Post-mortem toxicological analyses of blood samples from 107 patients receiving opioid agonist treatment: substances detected and pooled opioid and benzodiazepine concentrations. Addiction. doi: 10.1111/add.15211, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

