Preliminary evaluation of the FastCAP for users of the Nurotron cochlear implant
- PMID: 39840009
- PMCID: PMC11748202
- DOI: 10.3389/fnins.2024.1523212
Preliminary evaluation of the FastCAP for users of the Nurotron cochlear implant
Abstract
Background: Electrically evoked compound action potential (ECAP) can be used to measure the auditory nerve's response to electrical stimulation in cochlear implant (CI) users. In the Nurotron CI system, extracting the ECAP waveform from the stimulus artifact is time-consuming.
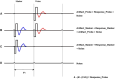
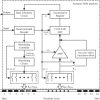
Method: We developed a new paradigm ("FastCAP") for use with Nurotron CI devices. In electrically evoked compound action potential in fast mode (FastCAP), N recordings are averaged directly on the CI hardware before data transmission, significantly reducing data transmission time. FastCAPs and ECAPs were measured across five electrodes and four stimulation levels per electrode. The FastCAP stimulation rate (33.3 Hz) is also faster than the ECAP rate (2.5 Hz).
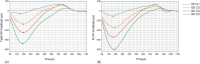
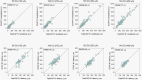
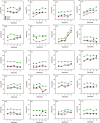
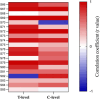
Results: Results showed strong correlations between ECAPs and FastCAPs for N1 latency (r = 0.84, p < 0.001) and N1 amplitude (r = 0.97, p < 0.001). Test-retest reliability for FastCAPs was also high, with intraclass correlation coefficients of r = 0.87 for N1 latency (p < 0.001) and r = 0.96 for N1 amplitude (p < 0.001). The mean test time was 46.9 ± 1.4 s for the FastCAP and 340.3 ± 6.3 s for the ECAP. The FastCAP measurement time was significantly shorter than the ECAP measurement time (W = -210.0, p < 0.001). FastCAP thresholds were significantly correlated with behavioral thresholds in 7/20 participants and with comfortable loudness levels in 11/20 participants. The time required to measure FastCAPs was significantly lower than that for ECAPs. The FastCAP paradigm maintained the accuracy and reliability the ECAP measurements while offering a significant reduction in time requirements.
Conclusion: This preliminary evaluation suggests that the FastCAP could be an effective clinical tool to optimize CI processor settings (e.g., threshold stimulation levels) in users of the Nurotron CI device.
Keywords: Nurotron; cochlear implant; comfortable loudness level; electrically evoked compound action potential; threshold level.
Copyright © 2025 Yang, Huang, Fu, Galvin, Chen, Liu and Tao.
Conflict of interest statement
Q-JF and SH have financial interests at Nurotron Biotechnology Co., Ltd., a medical device company that designs, develops, and markets CI systems. These affiliations are disclosed to ensure transparency and integrity in our research. We confirm that these affiliations did not influence the design, conduct, or reporting of the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer HJ declared a past co-authorship with the author SH.
Figures


References
LinkOut - more resources
Full Text Sources

