Therapeutic effects of composite probiotics derived from fermented camel milk on metabolic dysregulation and intestinal barrier integrity in type 2 diabetes rats
- PMID: 39840100
- PMCID: PMC11747018
- DOI: 10.3389/fphar.2024.1520158
Therapeutic effects of composite probiotics derived from fermented camel milk on metabolic dysregulation and intestinal barrier integrity in type 2 diabetes rats
Abstract
Background: In the Kazakh community of Xinjiang, China, fermented camel milk has been traditionally used to manage diabetes. This study evaluates the effects of composite probiotics derived from fermented camel milk (CPCM) on metabolic disturbances in a rat model of Type 2 diabetes (T2DM).
Methods: T2DM was induced in Wistar rats using streptozotocin. Experimental groups included a diabetic control, Metformin, and low- and high-dose CPCM. Measurements over 6 weeks included body weight (BW), fasting blood glucose (FBG), oral glucose tolerance test (OGTT), glycated hemoglobin (HbA1c), C-peptide (CP), lipid profiles, inflammatory markers, fecal short-chain fatty acids (SCFAs), and tight junction protein expression in colonic tissues.
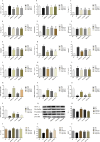
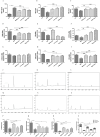
Results: High-dose CPCM significantly increased BW by 22.2% (p < 0.05) and reduced FBG by 6.5 mmol/L (p < 0.001). The OGTT AUC decreased by 40.1% (p < 0.001), and HbA1c levels fell by 22.9% (p < 0.01). CP levels rose by 21.8% (p < 0.05). Lipid profiles improved: TC decreased by 40.0%, TG by 17.1%, and LDL-C by 30.4% (all p < 0.001). Fecal SCFAs, including acetate (75.4%, p < 0.001), methyl acetate (18.9%, p < 0.05), and butyrate (289.9%, p < 0.001), increased, with total SCFAs rising by 89.7% (p < 0.001). Inflammatory markers IL-1β (12.7%, p < 0.01), TNF-α (16.7%, p < 0.05), and IL-6 (17.3%, p < 0.01) were significantly reduced. Tight junction protein expression (ZO-1, occludin, claudin-1) and mucin (MUC2) in colonic tissues increased (p < 0.05). CPCM treatment also reduced serum total bile acids by 24.9%, while hepatic and fecal bile acids increased by 114.0% and 37.8% (all p < 0.001). CPCM lowered serum DAO, D-lactate, and LPS levels (all p < 0.001). mRNA levels of TGR5 and CYP7A1 in the liver, and TGR5 and FXR in the colon, were markedly elevated (all p < 0.001). Histological examinations revealed reduced pancreatic inflammation and hepatic steatosis, with restored colonic structure.
Conclusion: CPCM treatment significantly improved metabolic dysregulation in the T2DM rat model, reducing blood glucose and lipid levels, enhancing intestinal barrier function, and increasing insulin secretion. These findings highlight the therapeutic potential of CPCM in T2DM management and probiotics' role in metabolic health.
Keywords: colon health; composite probiotics; fermented camel milk; lactic acid bacteria; tight junction proteins; type 2 diabetes; yeast.
Copyright © 2025 Manaer, Sailike, Sun, Yeerjiang and Nabi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adler A. I., Coleman R. L., Leal J., Whiteley W. N., Clarke P., Holman R. R. (2024). Post-trial monitoring of a randomised controlled trial of intensive glycaemic control in type 2 diabetes extended from 10 years to 24 years (UKPDS 91). Lancet 404, 145–155. 10.1016/S0140-6736(24)00537-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

