Early Cold-stored Platelet Transfusion following Traumatic Brain Injury: A Randomized Clinical Trial
- PMID: 39840438
- PMCID: PMC11974628
- DOI: 10.1097/SLA.0000000000006640
Early Cold-stored Platelet Transfusion following Traumatic Brain Injury: A Randomized Clinical Trial
Abstract
Objective: To determine the feasibility, efficacy, and safety of cold storage compared with room temperature (RT) platelet transfusion in patients with traumatic brain injury (TBI).
Background: Data demonstrating the safety and efficacy of cold-stored platelet (CSP) transfusion are lacking after TBI.
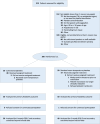
Methods: A phase 2, randomized, open-label clinical trial was performed at a single U.S. trauma center. Traumatic brain-injured patients with positive brain imaging and a need for platelet transfusion received up to 2 apheresis units of CSPs stored out to 14 days versus standard care RT platelet transfusion. The primary outcome was feasibility and the principal clinical outcome for efficacy and safety was the 6-month Glasgow Coma Scale-extended score.
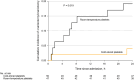
Results: The 6-month Glasgow Outcome Scale-extended score distributions were not different across cold stored and RT platelet arms (odds ratio: 1.58, 95% CI: 0.71 to 3.54, P = 0.27). A lower rate of neurosurgical craniotomy/craniectomy was found for those receiving CSPs (difference: -14.4%, 95% CI: -26.5% to -2.3%, P = 0.03). Adverse event rates did not differ across groups. The storage age of the cold-stored product was not associated with outcome differences.
Conclusions: In brain-injured patients requiring platelet transfusion, early CSP transfusion is feasible and did not result in improved 6-month Glasgow Coma Scale-extended scores. Early CSP transfusion was associated with a lower rate of neurosurgical operative intervention without an increase in adverse events. The storage age of the CSP product was not associated with outcome differences. Future phase 3 clinical trials are required to determine clinical outcome differences and safety attributable to CSP transfusion after TBI.
Keywords: antiplatelet medication; cold-stored platelets; randomized; traumatic brain injury.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.D.N. has received grants from the National Institutes of Health, Department of Defense, DARPA, Haemonetics, Alexion and Instrumentation Laboratories, honoraria for lectures from Haemonetics and Takeda, support for attending meetings and/or travel from Takeda; participates on a Data Safety Monitoring Board or Advisory Board from NHLBI CONNECTS Steering Committee; is the Chief Medical Officer, Haima Therapeutics, and has Patents planned, issued or pending (US Patent 11,408.844; US Patent 9.072,760), outside the submitted work. D.O.O. reports funding from NIH, DoD, and Abbott. F.X.G. reports grants from the DoD. P.C.S. reports personal fees from Hemanext, Cerus, participates in advisory board for Octapharma and Haima, and is the Co-Founder and Chief Medical Officer of Kalocyte, outside the submitted work. S.R.W. reports grants from the DoD. J.L.S. reports grants from the DoD. The remaining authors report no conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

