A Baicalin-Based Functional Polymer in Dynamic Reversible Networks Alleviates Osteoarthritis by Cellular Interactions
- PMID: 39840483
- PMCID: PMC11904974
- DOI: 10.1002/advs.202410951
A Baicalin-Based Functional Polymer in Dynamic Reversible Networks Alleviates Osteoarthritis by Cellular Interactions
Abstract
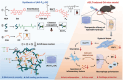
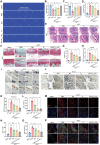
Osteoarthritis (OA) is increasingly recognized as a whole-organ disease predominantly affecting the elderly, characterized by typical alterations in subchondral bone and cartilage, along with recurrent synovial inflammation. Despite the availability of various therapeutics and medications, a complete resolution of OA remains elusive. In this study, novel functional hydrogels are developed by integrating natural bioactive molecules for OA treatment. Specifically, baicalin (Bai) is combined with 2-hydroxyethyl acrylate (HEA) to form a polymerizable monomer (HEA-Bai) through esterification, which is subjected to reversible addition-fragmentation chain transfer (RAFT) polymerization to produce Bai-based polymer (Pm). These macromolecules are incorporated into Schiff-base hydrogels, which demonstrate excellent mechanical properties and self-healing performance. Notably, the Bai-based formulations are taken up by fibroblast-like synoviocytes (FLSs), where they regulate glycolysis. Mechanistically, inhibition of yes-associated protein 1 (YAP1) by the formulations suppressed the FLSs glycolysis and reduced the secretion of inflammatory factors, including interleukin 1β (IL-1β), IL-6, and IL-8. Furthermore, the functional hydrogel (AG-Pm)-OC, severing as a lubricant and nutrient, prolonged joint retention of Bai, thereby reducing cartilage degradation and synovial inflammation. Meanwhile, (AG-Pm)-OC alleviated joint pain by targeting the YAP1 signaling and inhibiting macrophage recruitment and polarization. Taken together, this flavonoid-based injectable hydrogel exhibits enhanced biocompatibility and efficacy against OA.
Keywords: Bai‐based polymer; Schiff‐base hydrogel; anti‐inflammation; glycolysis; osteoarthritis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Obeidat A. M., Wood M. J., Adamczyk N. S., Ishihara S., Li J., Wang L., Ren D., Bennett D. A., Miller R. J., Malfait A.‐M., Miller R. E., Nat. Commun. 2023, 14, 2479; - PMC - PubMed
- b) Wang L., Chen X., Wang S., Ma J., Yang X., Chen H., Xiao J., Adv. Healthcare Mater. 2024, 13, 2302833;
- c) Fu W., Vasylyev D., Bi Y., Zhang M., Sun G., Khleborodova A., Huang G., Zhao L., Zhou R., Li Y., Liu S., Cai X., He W., Cui M., Zhao X., Hettinghouse A., Good J., Kim E., Strauss E., Leucht P., Schwarzkopf R., Guo E. X., Samuels J., Hu W., Attur M., Waxman S. G., Liu C.‐j., Nature 2024, 625, 557. - PMC - PubMed
-
- Van Linthout S., Miteva K., Tschöpe C., Cardiovasc. Res. 2014, 102, 258. - PubMed
MeSH terms
Substances
Grants and funding
- 2024A04J6408/Science and Technology Projects in Guangzhou
- 2023A03J1014/Science and Technology Projects in Guangzhou
- 82072470/National Natural Science Foundation of China
- 2023M741385/China Postdoctoral Science Foundation
- K. C. Wong Education Foundation
- U24A6013/Joint Funds of the National Natural Science Foundation of China
- JNU1AF-CFTP-2022-a01221/Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University
- 2023B1515020007/Basic and Applied Basic Research Foundation of Guangdong Province
- 2021A1515012154/Basic and Applied Basic Research Foundation of Guangdong Province
- 2019A1515011082/Basic and Applied Basic Research Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
