Nuclear Condensates of WW Domain-Containing Adaptor With Coiled-Coil Regulate Mitophagy via Alternative Splicing
- PMID: 39840526
- PMCID: PMC11904943
- DOI: 10.1002/advs.202406759
Nuclear Condensates of WW Domain-Containing Adaptor With Coiled-Coil Regulate Mitophagy via Alternative Splicing
Abstract
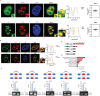
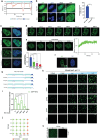
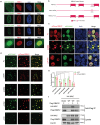
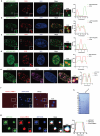
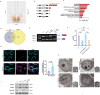
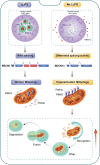
Biomolecular condensates segregate nuclei into discrete regions, facilitating the execution of distinct biological functions. Here, it is identified that the WW domain containing adaptor with coiled-coil (WAC) is localized to nuclear speckles via its WW domain and plays a pivotal role in regulating alternative splicing through the formation of biomolecular condensates via its C-terminal coiled-coil (CC) domain. WAC acts as a scaffold protein and facilitates the integration of RNA-binding motif 12 (RBM12) into nuclear speckles, where RBM12 potentially interacts with the spliceosomal U5 small nuclear ribonucleoprotein (snRNP). Importantly, knockdown of RBM12, or deletion of the WAC CC domain led to altered splicing outcomes, resulting in an elevated level of BECN1-S, the short splice variant of BECN1 that is shown to upregulate mitophagy. Thus, the findings reveal a previously unrecognized mechanism for the nuclear regulation of mitochondrial function through liquid-liquid phase separation (LLPS) and provide insights into the pathogenesis of WAC-related disorders.
Keywords: WAC; mitophagy; nuclear speckles; phase‐separated biomolecular condensates; pre‐mRNA splicing; snRNP.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
