Peptide-Drug Conjugate for Therapeutic Reprogramming of Tumor-Associated Macrophages in Breast Cancer
- PMID: 39840532
- PMCID: PMC11904948
- DOI: 10.1002/advs.202410288
Peptide-Drug Conjugate for Therapeutic Reprogramming of Tumor-Associated Macrophages in Breast Cancer
Abstract
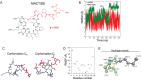
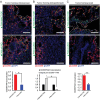
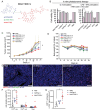
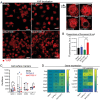
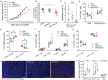
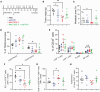
In triple-negative breast cancer (TNBC), pro-tumoral macrophages promote metastasis and suppress the immune response. To target these cells, a previously identified CD206 (mannose receptor)-binding peptide, mUNO was engineered to enhance its affinity and proteolytic stability. The new rationally designed peptide, MACTIDE, includes a trypsin inhibitor loop, from the Sunflower Trypsin Inhibitor-I. Binding studies to recombinant CD206 revealed a 15-fold lower KD for MACTIDE compared to parental mUNO. Mass spectrometry further demonstrated a 5-fold increase in MACTIDE's half-life in tumor lysates compared to mUNO. Homing studies in TNBC-bearing mice shows that fluorescein (FAM)-MACTIDE precisely targeted CD206+ tumor-associated macrophages (TAM) upon intravenous, intraperitoneal, and even oral administration, with minimal liver accumulation. MACTIDE was conjugated to Verteporfin, an FDA-approved photosensitizer and YAP/TAZ pathway inhibitor to create the conjugate MACTIDE-V. In the orthotopic 4T1 TNBC mouse model, non-irradiated MACTIDE-V-treated mice exhibited anti-tumoral effects comparable to those treated with irradiated MACTIDE-V, with fewer signs of toxicity, prompting further investigation into the laser-independent activity of the conjugate. In vitro studies using bone marrow-derived mouse macrophages showed that MACTIDE-V excluded YAP from the nucleus, increased phagocytic activity, and upregulated several genes associated with cytotoxic anti-tumoral macrophages. In mouse models of TNBC, MACTIDE-V slowed primary tumor growth, suppressed lung metastases, and increased markers of phagocytosis and antigen presentation in TAM and monocytes, increasing the tumor infiltration of several lymphocyte subsets. MACTIDE-V is proposed as a promising peptide-drug conjugate for modulating macrophage function in breast cancer immunotherapy.
Keywords: CD206; peptide‐drug conjugate; targeting peptides; triple negative breast cancer; tumor‐associated macrophages.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
