Antigen 43 associated with Escherichia coli membrane vesicles contributes to bacterial cell association and biofilm formation
- PMID: 39840972
- PMCID: PMC11878089
- DOI: 10.1128/spectrum.01890-24
Antigen 43 associated with Escherichia coli membrane vesicles contributes to bacterial cell association and biofilm formation
Abstract
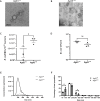
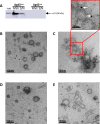
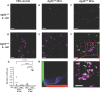
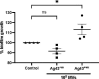
Bacterial membrane vesicles (MVs) are produced by all bacteria and contribute to numerous bacterial functions due to their ability to package and transfer bacterial cargo. In doing so, MVs have been shown to facilitate horizontal gene transfer, mediate antimicrobial activity, and promote biofilm formation. Uropathogenic Escherichia coli is a pathogenic Gram-negative organism that persists in the urinary tract of its host due to its ability to form persistent, antibiotic-resistant biofilms. The formation of these biofilms is dependent upon proteins such as Antigen 43 (Ag43), which belongs to the widespread Autotransporter group of bacterial surface proteins. In E. coli, the autotransporter Ag43 has been shown to contribute to bacterial cell aggregation and biofilm formation via self-association of Ag43 between neighboring Ag43-expressing bacteria. As MVs package bacterial proteins, we investigated whether MVs produced by E. coli contained Ag43, and the ability of Ag43-expressing MVs to facilitate cell aggregation and biofilm formation. We showed that Ag43 expressing E. coli produced MVs that contained Ag43 on their surface and had an enhanced ability to bind to E. coli bacteria. Furthermore, we demonstrated that the addition of Ag43-containing MVs to Ag43-expressing E. coli significantly enhanced biofilm formation. These findings reveal the contribution of MVs harboring autotransporters in promoting bacterial aggregation and enhancing biofilm formation, highlighting the impact of MVs and their specific composition to bacterial adaptation and pathogenesis.IMPORTANCEAutotransporter proteins are the largest family of outer membrane and secreted proteins in Gram-negative bacteria which contribute to pathogenesis by promoting aggregation, biofilm formation, persistence, and cytotoxicity. Although the roles of bacterial autotransporters are well known, the ability of bacterial membrane vesicles (MVs) naturally released from the surface of bacteria to contain autotransporters and their role in promoting virulence remains less investigated. Our findings reveal that MVs produced by E. coli contain the autotransporter protein Ag43. Furthermore, we show that Ag43-containing MVs function to enhance bacterial cell interactions and biofilm formation. By demonstrating the ability of MVs to carry functional autotransporter adhesins, this work highlights the importance of MVs in disseminating autotransporters beyond the bacterial cell membrane to ultimately promote cellular interactions and enhance biofilm development. Overall, these findings have significant implications in furthering our understanding of the numerous ways in which MVs can facilitate bacterial persistence and pathogenesis.
Keywords: Antigen 43; autotransporters; bacterial membrane vesicles; biofilm; outer membrane vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Identification of antigen Ag43 in uropathogenic Escherichia coli Dr+ strains and defining its role in the pathogenesis of urinary tract infections.Microbiology (Reading). 2015 May;161(Pt 5):1034-1049. doi: 10.1099/mic.0.000072. Epub 2015 Mar 5. Microbiology (Reading). 2015. PMID: 25743156
-
Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract.Infect Immun. 2007 Jul;75(7):3233-44. doi: 10.1128/IAI.01952-06. Epub 2007 Apr 9. Infect Immun. 2007. PMID: 17420234 Free PMC article.
-
Impact of O-glycosylation on the molecular and cellular adhesion properties of the Escherichia coli autotransporter protein Ag43.Int J Med Microbiol. 2009 Aug;299(6):389-401. doi: 10.1016/j.ijmm.2009.01.001. Epub 2009 Feb 20. Int J Med Microbiol. 2009. PMID: 19230760
-
Self-associating autotransporters, SAATs: functional and structural similarities.Int J Med Microbiol. 2006 Aug;296(4-5):187-95. doi: 10.1016/j.ijmm.2005.10.002. Int J Med Microbiol. 2006. PMID: 16600681 Review.
-
Autotransporter Adhesins in Escherichia coli Pathogenesis.Proteomics. 2017 Dec;17(23-24). doi: 10.1002/pmic.201600431. Epub 2017 Oct 12. Proteomics. 2017. PMID: 28665015 Review.
Cited by
-
ESBL-Producing Escherichia coli and Klebsiella pneumoniae Exhibit Divergent Paths During In-Human Evolution Towards Carbapenem Resistance.Microorganisms. 2025 Jun 14;13(6):1387. doi: 10.3390/microorganisms13061387. Microorganisms. 2025. PMID: 40572275 Free PMC article.
References
-
- Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, Hutton ML, Davies JK, Crack PJ, Hertzog PJ, Philpott DJ, Girardin SE, Whitchurch CB, Ferrero RL. 2010. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol 12:372–385. doi:10.1111/j.1462-5822.2009.01404.x - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

