Autophagy-mediated TET2 degradation by ALV-J Env protein suppresses innate immune activation to promote viral replication
- PMID: 39840982
- PMCID: PMC11852998
- DOI: 10.1128/jvi.02003-24
Autophagy-mediated TET2 degradation by ALV-J Env protein suppresses innate immune activation to promote viral replication
Abstract
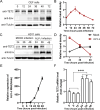
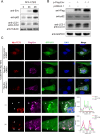
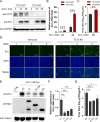
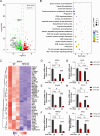
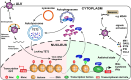
Avian leukosis virus subgroup J (ALV-J) poses a significant threat to the poultry industry; yet, our understanding of its replication and pathogenic mechanisms is limited. The Ten-Eleven Translocation 2 (TET2) is an indispensable regulatory factor in active DNA demethylation and immune response regulation. This study reports a significant and time-dependent decrease in TET2 levels following ALV-J infection and shows that the reduction of TET2 protein is mediated by the autophagy pathway. Mechanistically, we found that the accumulation of the Env protein at the late stages of ALV-J infection triggers autophagy, which, in turn, causes the TET2 protein to be exported from the nucleus and subsequently degraded in the cytoplasm. Using CRISPR-Cas9 technology, we generated TET2-deficient chicken macrophages that exhibited increased susceptibility to ALV-J replication, which was reversed by TET2 overexpression. In addition, transcriptome analysis revealed that the absence of TET2 in chicken macrophages impairs the expression of numerous cytokines and innate immune-related genes, particularly those downregulated genes enriched in the RIG-I and Toll-like signaling pathways, leading to enhanced replication of ALV-J in vitro. In summary, our research identifies TET2 as an ALV-J restriction factor. However, ALV-J exploits the autophagy machinery to promote the degradation of TET2 protein, thereby disrupting the host's innate immune responses for viral replication.
Importance: ALV-J is a carcinogenic retrovirus that plays a critical role in avian leukosis, primarily affecting chickens. Infection with ALV-J leads to decreased production performance, compromised immune function, and the development of tumors, such as myelocytoma. Currently, there are no effective treatments for ALV-J, making the control of outbreaks a significant challenge with severe economic consequences for the poultry industry. The Env protein of ALV-J has been implicated in the virus's pathogenicity. Our study shows that ALV-J infection induces autophagy in host cells through its Env protein, leading to the autophagic degradation of TET2, a key epigenetic regulator. The loss of TET2 in macrophages results in the downregulation of innate immune-related gene expression, thereby promoting viral replication. This is the first report to elucidate the role of the ALV-J Env protein in immune suppression via TET2 autophagic degradation during ALV-J infection, providing new insights into the mechanisms of viral immune evasion.
Keywords: Env; TET2; autophagic degradation; avian leukosis virus subgroup J; immunosuppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yu M, Zhang Y, Zhang L, Wang S, Liu Y, Xu Z, Liu P, Chen Y, Guo R, Meng L, Zhang T, Fan W, Qi X, Gao L, Zhang Y, Cui H, Gao Y. 2024. N123I mutation in the ALV-J receptor-binding domain region enhances viral replication ability by increasing the binding affinity with chNHE1. PLoS Pathog 20:e1011928. doi: 10.1371/journal.ppat.1011928 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

