Evaluation of antimicrobial susceptibility testing methods for Burkholderia cepacia complex isolates from people with and without cystic fibrosis
- PMID: 39840992
- PMCID: PMC11837569
- DOI: 10.1128/jcm.01480-24
Evaluation of antimicrobial susceptibility testing methods for Burkholderia cepacia complex isolates from people with and without cystic fibrosis
Abstract
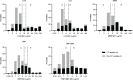
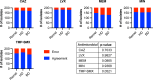
The Burkholderia cepacia complex (BCC) is a group of Gram-negative bacteria that cause opportunistic infections, most notably in people with cystic fibrosis (CF), and have been associated with outbreaks caused by contaminated medical products. Antimicrobial susceptibility testing (AST) is often used to guide treatment for BCC infections, perhaps most importantly in people with CF who are being considered for lung transplant. However, recent studies have highlighted problems with AST methods. Here, we address limitations from previous studies to further evaluate BCC AST methods. We assessed the performance of reference broth microdilution (BMD), disk diffusion (DD) using Mueller-Hinton agar (MHA) from three manufacturers, agar dilution (AD), and gradient diffusion (ETEST) for ceftazidime (CAZ), levofloxacin (LVX), meropenem (MEM), minocycline (MIN), and trimethoprim-sulfamethoxazole (TMP-SMX) on a set of 205 BCC isolates. The isolate set included 100 isolates from people with CF and 105 isolates from people without CF from a variety of sources, which enabled us to systematically evaluate whether specimen source impacts AST performance. For all BCC isolates, BMD reproducibility was 93%, 98%, 99%, 98%, and 96% for CAZ, LVX, MEM, MIN, and TMP-SMX, respectively. Using BMD as the comparator method, we show that DD, AD, and ETEST perform poorly, with neither MHA manufacturer nor specimen source significantly impacting method performance. Based on our data, we recommend that routine AST should not be performed for BCC isolates. If a provider requests AST, clinical microbiology laboratories should perform Clinical and Laboratory Standards Institute reference methodology for BMD (stored frozen) and report MIC only.IMPORTANCEAntimicrobial susceptibility testing for the Burkholderia cepacia complex (BCC) is often used to determine eligibility for lung transplant in people with cystic fibrosis. However, problems with method performance have been reported. Here, we systematically evaluate the performance of reference broth microdilution, disk diffusion, agar dilution, and gradient diffusion (ETEST) for BCC organisms isolated from people with and without cystic fibrosis. We show that broth microdilution reproducibility is acceptable for levofloxacin, meropenem, minocycline, and trimethoprim-sulfamethoxazole, while ceftazidime was just below the acceptability cut-off. Regardless of specimen source, the results from disk diffusion, agar dilution, and ETEST do not correlate with broth microdilution. Based on these findings, we recommend that antimicrobial susceptibility testing should not be routinely performed for BCC, and if requested by the provider, only broth microdilution following Clinical and Laboratory Standards Institute guidelines should be used. Providers should be aware of the significant limitations of antimicrobial susceptibility testing methods for BCC.
Keywords: Burkholderia cepacia complex; antimicrobial agents; cystic fibrosis; susceptibility testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- El Chakhtoura NG, Saade E, Wilson BM, Perez F, Papp-Wallace KM, Bonomo RA. 2017. A 17-year nationwide study of Burkholderia cepacia complex bloodstream infections among patients in the United States Veterans Health Administration. Clin Infect Dis 65:1253–1259. doi: 10.1093/cid/cix559 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

