Aging platelets shift their hemostatic properties to inflammatory functions
- PMID: 39841014
- PMCID: PMC12002221
- DOI: 10.1182/blood.2024024901
Aging platelets shift their hemostatic properties to inflammatory functions
Abstract
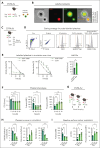
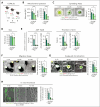
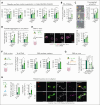
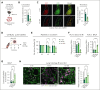
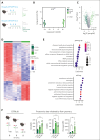
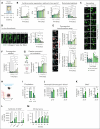
Platelets are crucial players in hemostasis and thrombosis but also contribute to immune regulation and host defense, using different receptors, signaling pathways, and effector functions, respectively. Whether distinct subsets of platelets specialize in these diverse tasks is insufficiently understood. Here, we used a pulse-labeling method in Mus musculus models for tracking in vivo platelet aging and its functional implications. Using in vitro and in vivo assays, we reveal that young, reticulated platelets show heightened responses in the setting of clot formation, with corresponding, increased responses to agonists, adhesion, and retractile function. Unexpectedly, aged platelets lose their hemostatic proficiency but are more prone to react to inflammatory challenge: compared with reticulated platelets, this cohort was more likely to form platelet-leukocyte aggregates and showed increased adhesion to neutrophils in vitro, as well as enhanced bactericidal function. In vivo, this was reflected in increased pulmonary recruitment of aged platelets in an acute lung injury model. Proteomic analyses confirmed the upregulation of immune pathways in this cohort, including enhanced procoagulant function. In mouse models of prolonged platelet half-life, this resulted in increased pulmonary leukocyte infiltration and inflammation upon acute lung injury. Similarly, human platelet concentrates decreased their hemostatic function and elevated their putative immunomodulatory potential in vitro over time, and in a mouse model of platelet transfusion, aged platelet concentrates resulted in augmented inflammation. In summary, we show that platelets exhibit age-dependent phenotypic shifts, allowing them to fulfill their diverse tasks in the vasculature. Because functional alterations of aging platelets extend to platelet concentrates, this may hold important implications for transfusion medicine.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
"When I'm 64": functional shift of aging platelets.Blood. 2025 Apr 3;145(14):1447-1449. doi: 10.1182/blood.2024027996. Blood. 2025. PMID: 40178850 No abstract available.
References
-
- Nicolai L, Massberg S. Platelets as key players in inflammation and infection. Curr Opin Hematol. 2020;27(1):34–40. - PubMed
-
- Kaiser R, Escaig R, Nicolai L. Hemostasis without clot formation–how platelets guard the vasculature in inflammation, infection, and malignancy. Blood. 2023;142(17):1413–1425. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

