DNA-Dependent Protein Kinase Catalytic Subunit Prevents Ferroptosis in Retinal Pigment Epithelial Cells
- PMID: 39841110
- PMCID: PMC11756607
- DOI: 10.1167/iovs.66.1.50
DNA-Dependent Protein Kinase Catalytic Subunit Prevents Ferroptosis in Retinal Pigment Epithelial Cells
Abstract
Purpose: The purpose of this study was to investigate the activated core kinases involved in the DNA damage responses (DDR) during ferroptosis of retinal pigment epithelial (RPE) cells in vitro and their regulatory effects on ferroptosis.
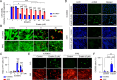
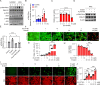
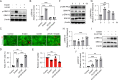
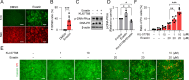
Methods: Ferroptosis was induced by erastin in induced RPE (iRPE) cells derived from human umbilical cord mesenchymal stem cells (hUCMSCs), hUCMSCs, and induced pluripotent stem cell-derived RPE (iPSC-RPE) cells. CCK8 was employed to measure the cell viability. Calcein/PI staining was used to detect the ferroptotic cells. The γ-H2AX, 8-oxoG, and phosphorylated DNA-dependent protein kinase catalytic subunit (DNA-PKcs) were determined through immunostaining. The phosphorylation of DNA-PKcs and ERK1/2 was determined by Western blotting. Lipid peroxides were detected by BODIPY581/591-C11 staining.
Results: The iRPE cells exhibited a stronger ability to resist ferroptosis compared to hUCMSCs. Ferroptosis induced DNA damage in cells, and DNA-PKcs was rapidly phosphorylated in iRPE cells on the treatment of erastin. In addition, inhibition of DNA-PKcs phosphorylation promoted ferroptosis in iRPE cells, suggesting that DNA-PKcs prevents ferroptosis. Meanwhile, DNA-PKcs inhibited ERK1/2 phosphorylation only at the early stage of ferroptosis induction, whereas ERK1/2 phosphorylation played a protective role in iRPE cells. Furthermore, erastin inducing DNA-PKcs phosphorylation and inhibition of its phosphorylation promoting ferroptosis were also verified in iPSC-RPE cells.
Conclusions: The present study elucidates that the key DDR kinase DNA-PKcs is activated and plays protective role during ferroptosis in RPE cells in vitro, which will provide new research targets and strategies for inhibiting ferroptosis in RPE cells.
Conflict of interest statement
Disclosure:
Figures

References
-
- Biesemeier A, Yoeruek E, Eibl O, Schraermeyer U. Iron accumulation in Bruch's membrane and melanosomes of donor eyes with age-related macular degeneration. Exp Eye Res. 2015; 137: 39–49. - PubMed
-
- Chao C, Yang K, He D, Yang B. Induction of ferroptosis by HO-1 contributes to retinal degeneration in mice with defective clearance of all-trans-retinal. Free Radic Biol Med. 2023; 194: 245–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

