Population-Based Age-Period-Cohort Analysis of Declining Human Papillomavirus Prevalence
- PMID: 39841153
- PMCID: PMC11998575
- DOI: 10.1093/infdis/jiaf032
Population-Based Age-Period-Cohort Analysis of Declining Human Papillomavirus Prevalence
Abstract
Background: Most countries in the world have launched human papillomavirus (HPV) vaccination programs, and declining HPV prevalences are reported. We aimed to disentangle the influences of calendar time, birth cohort, and age by analyzing HPV prevalences in the population-based cervical screening program using age-period-cohort modeling.
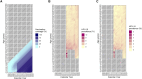
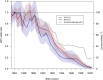
Methods: All 813 882 primary HPV-based cervical screening tests from women aged 23-64 years between 2014 and 2023 in the capital region of Sweden were identified in the Swedish National Cervical Screening Registry. The odds ratio (OR) of HPV-16/18 infection was estimated comparing birth cohorts to the unvaccinated 1984-born using an age-period-cohort model. The impact of changing HPV prevalences on the number needed to screen (NNS) to detect and prevent 1 cervical cancer case was calculated.
Results: HPV vaccination coverage was 82%-83% among women born in 1999-2000. Before 2019, the HPV-16/18 prevalence was highest among the youngest women. During 2020-2023 the prevalence consistently decreased among the birth cohorts offered organized school-based vaccination. There was a 98% decline in HPV-16 prevalence (OR, 0.02 [95% confidence interval {CI}, .01-.04]) and a 99% decline in HPV-18 prevalence (OR, 0.01 [95% CI, .00-.04]) among the 2000-born compared to the 1984-born. The declining HPV-16/18 prevalences resulted in major increases in the NNS to detect and to prevent 1 case of cervical cancer.
Conclusions: The declines of HPV-16/18 were considerably larger than the vaccination coverage, suggesting herd immunity. The changing epidemiology of HPV types impacts screening needs, necessitating updated screening programs.
Keywords: HPV; HPV elimination; HPV prevalence; HPV vaccination; cervical screening; herd protection.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. W. and S. N. K. have participated in other studies (unrelated to this study) funded by MSD regarding HPV vaccination evaluation in Sweden. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- International Agency for Research on Cancer (IARC) . Biological agents. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 100B. Lyon, France: IARC, 2012.
-
- Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

