Ravulizumab demonstrates long-term efficacy, safety and favorable patient survival in patients with paroxysmal nocturnal hemoglobinuria
- PMID: 39841198
- PMCID: PMC11868214
- DOI: 10.1007/s00277-025-06193-5
Ravulizumab demonstrates long-term efficacy, safety and favorable patient survival in patients with paroxysmal nocturnal hemoglobinuria
Abstract
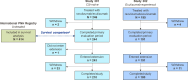
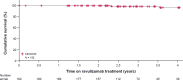
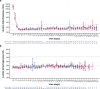
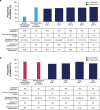
Ravulizumab is a second-generation complement component 5 (C5) inhibitor (C5i) approved for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) following positive results from two pivotal trials in patients with PNH originally naive to C5i treatment and eculizumab-experienced patients with PNH. In both trials, after the 26week primary evaluation period, all patients received ravulizumab for up to 6 years. To report ravulizumab treatment outcomes in patients with PNH originally naive to C5i treatment and eculizumab-experienced patients with PNH treated for up to 6 years. Originally C5i-naive (N = 244) and eculizumab-experienced (N = 191) patients with PNH continued ravulizumab treatment for up to 6 years. Major adverse vascular events (MAVEs; including thrombotic events [TEs]) and survival are reported, including a comparison of survival with untreated patients from the International PNH Registry. Laboratory parameters for intravascular hemolysis (IVH) are also described. For up to 6 years (1468.0 patient-years of exposure), ravulizumab provided durable control of terminal complement activity and IVH, resulting in a low incidence of MAVEs (including TEs) reported (MAVE rate: 0.7-1.4 per 100 patient-years) and, compared with untreated patients from the International PNH Registry, reduced the risk of mortality by five-fold. The few breakthrough IVH events reported (N = 122) were commonly associated with complement-amplifying conditions, and only two events (1.8%) were associated with suboptimal inhibition of C5 (i.e. serum free C5 ≥ 0.5 µg/mL). These results support the long-term use of ravulizumab as the first-line treatment of choice for patients with PNH. Trial registration details: NCT01374360; registered: October 29, 2004; NCT02946463; registered: October 27, 2016; NCT03056040; registered: June 05, 2017.
Keywords: Complement inhibitor; Intravascular hemolysis; Lactate dehydrogenase; Paroxysmal nocturnal hemoglobinuria; Ravulizumab; Survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The protocols for study 301, study 302 and the International PNH Registry were approved by the institutional review board or independent ethics committee at each participating center. Each study was conducted in accordance with the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines. Written informed consent was provided by all patients. Competing interests: AK has received honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Agios, Celgene/BMS, Novartis, Pfizer, Roche, Samsung, and Sobi, is on the board of directors or is an advisory board member for Alexion, AstraZeneca Rare Disease, Amgen, BioCryst, Celgene/BMS, Novartis, Regeneron, and Roche, and has received consulting fees from Alexion, AstraZeneca Rare Disease, Celgene/BMS, Novo Nordisk, Janssen Pharmaceuticals, Pfizer, Roche, Samsung, Sobi, and Novartis and research funding (to Institute) from Celgene and Novartis. RB has received research funding and consultancy fees from Alexion, AstraZeneca Rare Disease and honoraria from Alexion, AstraZeneca Rare Disease, American Society of Hematology, Indy Hematology Review, ISTH congress, and UpToDate. HS has received fees, research funding (to the University of Ulm) and travel support from Alexion, AstraZeneca Rare Disease, Novartis, and Sobi, and fees (to the University of Ulm) from Apellis, F. Hoffmann-La Roche, Novartis, Omeros, Sanofi, and Sobi. MG has received honoraria and advisory board fees from Alexion, AstraZeneca Rare Disease, and Sobi, consultancy fees and advisory board fees from BioCryst, and received consultancy fees from Regeneron Pharmaceuticals. They served as an advisory board member for Amgen and Novartis, and received financial support for Medscape education in PNH from Apellis. AR has received research funding from Roche, travel support from AbbVie, Alexion, AstraZeneca Rare Disease and Sobi, lecture honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Grifols, Roche, Sanofi, Sobi, and Novartis, and consultancy fees from Alexion, AstraZeneca Rare Disease, Apellis, BioCryst, Bioverativ, Kira, Novartis, Pfizer, and Sanofi. CP has received consulting fees, advisory board fees and research support from Alexion, AstraZeneca Rare Disease/AstraZeneca and Rigel, research support, advisory board fees and speaker’s bureau fees from Apellis, advisory board fees and research support from Sanofi, advisory board and speaker’s bureau fees from Sobi/Dova, research support from Argenx, Celgene, Incyte and Oscotec, and advisory board fees from Annexon Biosciences and Novartis. MO, JY, AP, and YP are current employee shareholders at Alexion, AstraZeneca Rare Disease. RN has received lecture fees from Alexion, AstraZeneca Rare Disease and has served as a member on an advisory board for Alexion, AstraZeneca Rare Disease, BioCryst, Novartis, and Sobi.KU has received research funding from Astellas, AbbVie, Alxion, Aperis, Bristol Myers Squibb, Celgene, Chugai, Daichi Sankyo, Eisai, Incyte, Kyowa Kirin, MSD, Nippon Shinyaku, Novartis, Ohara, Ono, Otsuka, Pfizer, Sanofi, Takeda, and Yakult, speaker’s bureau fees from Novartis, AbbVie, Alexion, Amgen, Asahi Kasei Pharma, AstraZeneca, Bristol Myers Squibb, Chugai, Eisai, Incyte, Kyowa Kirin, Meiji Seika Pharma, Nippon Shinyaku, Ono, Otsuka, PharmaEssentia, Sanofi, Takeda, and Janssen, and consulting bureau fees from Alnylam Japan, Chugai, Ohara, and Sobi. AK has received honoraria and research funding (to Pavlov University) from Alexion, AstraZeneca Rare Disease, Apellis, Generium and Sobi. SG has served as an advisory board member for and has received research funding from Agios, Alexion, AstraZeneca Rare Disease, and Novartis.WF has received honoraria from Alexion, AstraZeneca Rare Disease, Apellis Pharmaceuticals, BioCryst, Novartis, Roche and Sobi.RPdL has served as an advisory board member for and received consultancy fees, research funding, and speaker’s bureau fees from Alexion Pharmaceuticals Inc.JS has received consultancy fees, honoraria and research funding (to the Royal Melbourne Hospital) from Alexion, AstraZeneca Rare Disease, travel support from CSL, Novartis, Prevail Therapeutics, and Sobi and consultancy and speaker’s bureau fees from Takeda. JWL has received research funding from Achillion and Alexion, AstraZeneca Rare Disease, has served as advisory board member for and received honoraria from Alexion, AstraZeneca Rare Disease, and has received consultancy fees from Kyowa Kirin and Sanofi.
Figures

References
-
- Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T (1993) Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73(4):703–711. 10.1016/0092-8674(93)90250-T - PubMed
-
- Peacock-Young B, Macrae FL, Newton DJ, Hill A, Ariëns RAS (2018) The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica 103(1):9–17. 10.3324/haematol.2017.177618 - PubMed
-
- Kulasekararaj AG, Griffin M, Langemeijer S, Usuki K, Kulagin A, Ogawa M, Yu J, Mujeebuddin A, Nishimura JI, Lee JW, de Peffault R (2022) Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies. Eur J Haematol 109(3):205–214. 10.1111/ejh.13783 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

