Usefulness of serum HBV RNA levels for predicting antiviral response to entecavir treatment in patients with chronic hepatitis B
- PMID: 39841247
- PMCID: PMC11922970
- DOI: 10.1007/s00535-025-02211-5
Usefulness of serum HBV RNA levels for predicting antiviral response to entecavir treatment in patients with chronic hepatitis B
Abstract
Background: Hepatitis B virus (HBV) RNA is an important serum biomarker of hepatic covalently closed circular DNA (cccDNA) transcriptional activity; however, its clinical characteristics remain unclear. This study evaluated the clinical utility of HBV RNA levels in patients with chronic hepatitis B (CHB).
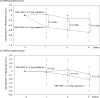
Methods: We studied 87 CHB patients with serum HBV DNA levels ≥ 5.0 log IU/mL who initiated entecavir (ETV) treatment between 2000 and 2018. Serum HBV RNA levels were measured at three-time points: before ETV treatment, at 12 weeks, and at 48 weeks after starting ETV treatment. Clinical markers associated with the antiviral effects of ETV treatment were analyzed.
Results: Serum HBV RNA levels decreased in both HBeAg-positive and -negative patients during the observation period. In HBeAg-positive patients, multivariable analysis showed that lower HBV RNA levels at 48 weeks of ETV treatment were independently associated with HBeAg seroconversion. Additionally, lower baseline HBV RNA levels significantly predicted virologic response in those patients. In contrast, among HBeAg-negative patients, lower HBV core-related antigen (HBcrAg) levels and the FIB-4 index were independently associated with virologic response. In HBeAg-positive patients, those with higher baseline HBV RNA levels showed a more significant reduction in hepatitis B surface antigen levels.
Conclusion: Serum HBV RNA levels predicted HBeAg seroconversion and early HBV DNA reduction in HBeAg-positive patients, while HBcrAg was significantly associated with virologic response in HBeAg-negative patients. These findings highlight the different predictive roles of HBV RNA and HBcrAg based on HBeAg status, which may provide individualized treatment strategies.
Keywords: Entecavir; HBV DNA; HBV RNA; HBeAg seroconversion; HBsAg.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare that they have no affiliations with or involvement in any organization with any financial or non-financial interests in the subject matter or materials discussed in this manuscript. Ethical approval and informed consent: All the participants provided written informed consent. This study was approved by the Ethics Committee of Hiroshima University, Hiroshima, Japan (Approved ID: E2016-0704). This study was conducted in accordance with the principles of the Declaration of Helsinki. All authors declare that they have no affiliations with or involvement in any organization with any financial or non-financial interests in the subject matter or materials discussed in this manuscript.
Figures


References
-
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Available online: https://www.who.int/publications/i/item/9789240027077 Accessed on 13 May 2024
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

