Traumatic brain injury causes early aggregation of beta-amyloid peptides and NOTCH3 reduction in vascular smooth muscle cells of leptomeningeal arteries
- PMID: 39841284
- PMCID: PMC11754316
- DOI: 10.1007/s00401-025-02848-9
Traumatic brain injury causes early aggregation of beta-amyloid peptides and NOTCH3 reduction in vascular smooth muscle cells of leptomeningeal arteries
Abstract
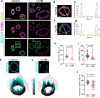
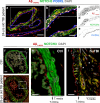
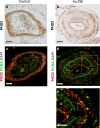
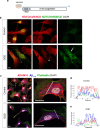
Traumatic brain injury (TBI) often leads to impaired regulation of cerebral blood flow, which may be caused by pathological changes of the vascular smooth muscle cells (VSMCs) in the arterial wall. Moreover, these cerebrovascular changes may contribute to the development of various neurodegenerative disorders such as Alzheimer's-like pathologies that include amyloid beta aggregation. Despite its importance, the pathophysiological mechanisms responsible for VSMC dysfunction after TBI have rarely been evaluated. Here, we show that acute human TBI resulted in early pathological changes in leptomeningeal arteries, closely associated with a decrease in VSMC markers such as NOTCH3 and alpha smooth muscle actin (α-SMA).These changes coincided with increased aggregation of variable-length amyloid peptides including Aβ1-40/42, Aβ1-16, and β-secretase-derived fragment (βCTF) (C99) caused by altered processing of amyloid precursor protein (APP) in VSMCs. The aggregation of Aβ1-40/42 peptides were also observed in the leptomeningeal arteries of young TBI patients. These pathological changes also included higher β-secretase (BACE1) when compared to α-secretase A Disintegrin And Metalloprotease 10 (ADAM10) expression in the leptomeningeal arteries, plausibly caused by hypoxia and oxidative stress as shown using human VSMCs in vitro. Importantly, BACE1 inhibition not only restored NOTCH3 signalling but also normalized ADAM10 levels in vitro. Furthermore, we found reduced ADAM10 activity and decreased NOTCH3, along with increased βCTF (C99) levels in mice subjected to an experimental model of TBI. This study provides evidence of early post-injury changes in VSMCs of leptomeningeal arteries that can contribute to vascular dysfunction and exacerbate secondary injury mechanisms following TBI.
Keywords: Amyloid beta; NOTCH3; Traumatic brain injury (TBI); Vascular smooth muscle cells; β-Secretase-derived fragment (βCTF) (C99).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that there is no conflict of interest.
Figures




Similar articles
-
Novel roles of Nrf3-Trim5 axis in vascular smooth muscle cell dysfunctions and neointimal hyperplasia.Cardiovasc Res. 2025 Jul 31;121(8):1282-1298. doi: 10.1093/cvr/cvaf084. Cardiovasc Res. 2025. PMID: 40377016 Free PMC article.
-
Altered amyloid-β structure markedly reduces gliosis in the brain of mice harboring the Uppsala APP deletion.Acta Neuropathol Commun. 2024 Feb 5;12(1):22. doi: 10.1186/s40478-024-01734-x. Acta Neuropathol Commun. 2024. PMID: 38317196 Free PMC article.
-
The amyloid precursor protein and its derived fragments concomitantly contribute to the alterations of mitochondrial transport machinery in Alzheimer's disease.Cell Death Dis. 2024 May 28;15(5):367. doi: 10.1038/s41419-024-06742-2. Cell Death Dis. 2024. PMID: 38806484 Free PMC article.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
Cited by
-
Potential Correlation Between Molecular Biomarkers and Oxidative Stress in Traumatic Brain Injury.Int J Mol Sci. 2025 Apr 18;26(8):3858. doi: 10.3390/ijms26083858. Int J Mol Sci. 2025. PMID: 40332547 Free PMC article. Review.
References
-
- Abrahamson EE, Ikonomovic MD (2020) Brain injury-induced dysfunction of the blood brain barrier as a risk for dementia. Exp Neurol 328:113257. 10.1016/j.expneurol.2020.113257 - PubMed
-
- Ashina H, Christensen RH, Al-Khazali HM, Iljazi A, Tolnai D, Eigenbrodt AK et al (2023) White matter hyperintensities and cerebral microbleeds in persistent post-traumatic headache attributed to mild traumatic brain injury: a magnetic resonance imaging study. J Headache Pain 24:15. 10.1186/s10194-023-01545-w - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

