Efficacy of Ceftazidime-avibactam in treating Gram-negative infections: a systematic review and meta-analysis
- PMID: 39841366
- PMCID: PMC11946964
- DOI: 10.1007/s10096-025-05044-5
Efficacy of Ceftazidime-avibactam in treating Gram-negative infections: a systematic review and meta-analysis
Abstract
Introduction: Ceftazidime-avibactam (CAZ-AVI) has emerged as a promising treatment option for Gram-negative infections, particularly those caused by CAZ-Non-Susceptible (NS) pathogens. This systematic review and meta-analysis aim to assess the efficacy and safety of CAZ-AVI in these challenging infections.
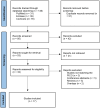
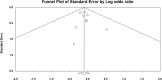
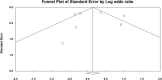
Methods: We systematically queried EMBASE, Cochrane CENTRAL, and PubMed/Medline for studies published until September 15, 2024. Randomized Controlled Trials (RCTs) evaluating CAZ-AVI against Gram-negative infections were included. A meta-analysis was performed to calculate pooled odds ratios (OR) for both clinical and microbiological success.
Results: A total of 146 studies were identified through database searches, leading to the inclusion of 17 studies. Among the efficacy studies for Gram-negative pathogens, there was no significant difference in clinical success rates for CAZ-AVI compared to comparators (pooled OR: 0.90, p = 0.22), and a non-significant increase in microbiological success was observed (pooled OR: 1.20, p = 0.41). In contrast, for CAZ-NS pathogens, six studies reported no significant difference in clinical cure rates (pooled OR: 0.77, p = 0.24), while four studies indicated a non-significant increase in microbiological cure rates (pooled OR: 1.83, p < 0.02).
Conclusions: This study suggests that CAZ-AVI is a viable option for treating Gram-negative infections, including CAZ-NS pathogens. While it has shown promising activity against these resistant pathogens, its clinical and microbiological success rates are comparable to other antibiotics in the overall analysis. However, CAZ-AVI may offer an advantage in managing resistant infections. These findings underscore the need to consider CAZ-AVI in treatment guidelines and emphasize the importance of antibiotic stewardship programs to optimize its use and prevent resistance. Ongoing monitoring of resistance patterns and patient outcomes is essential to ensure its long-term efficacy.
Keywords: Antibiotic resistance; CAZ-AVI; CAZ-non-susceptible pathogens; Ceftazidime-avibactam; Clinical success; Efficacy; Gram-negative infections; Meta-analysis; Microbiological success; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have consented to the publication of this manuscript. Competing interests: The authors declare no competing interests.
Figures
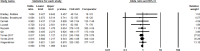
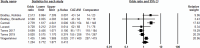
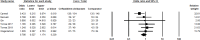
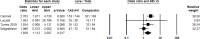
References
-
- Zhen S, Wang H, Feng S (2022) Update of clinical application in ceftazidime-avibactam for multidrug-resistant Gram-negative bacteria infections. Infection 50(6):1409–1423 - PubMed
-
- Díaz Santos E, Mora Jiménez C, del Río-Carbajo L, Vidal-Cortés P (2022) Treatment of severe multi-drug resistant < span class=elsevierStyleItalic>Pseudomonas aeruginosa infections. Med Intensiva (English Edition) 46(9):508–520 - PubMed
-
- Lodise T, O’Donnell JN, Raja S, Balevic S, Guptill J, Schwager N et al (2022) Safety of Ceftazidime-Avibactam (CZA) in combination with Aztreonam (ATM) in a phase I, open-label study in healthy adult subjects (COMBINE). Open Forum Infect Dis 9:S163–S4
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

