Did selective kinase inhibitors change the management of patients with radioiodine-refractory thyroid cancer?
- PMID: 39841677
- PMCID: PMC11831060
- DOI: 10.1530/ETJ-24-0332
Did selective kinase inhibitors change the management of patients with radioiodine-refractory thyroid cancer?
Abstract
Objective: To analyse at our institution the criteria for selecting a first-line therapy for patients with advanced radioiodine-refractory thyroid cancer and their clinical responses, safety and survival outcomes.
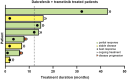
Patients and methods: We extracted data from 69 consecutive patients referred to Federico II University Hospital from September 2016 to September 2024, among whom 44 patients were treated with TKIs as first-line treatment and outside any clinical trial, and form the basis of this report.
Results: Thirty-one (71%) patients were treated with the antiangiogenesis inhibitor lenvatinib and 13 (29%) were treated with selective tyrosine kinase inhibitors (s-TKIs). Among the latter, eight patients were treated with dabrafenib + trametinib (DT), two patients were treated with selpercatinib because of contraindications to lenvatinib, and three patients received DT as redifferentiation therapy. A RECIST partial response was observed in 28% of patients treated with lenvatinib, in 63% of those treated with DT and in one of the two patients treated with selpercatinib. Grade ≥3 adverse events occurred in 13 (42%) patients treated with lenvatinib and only in 1 (9%) patient treated with DT. Progression-free survival (PFS) and overall survival rates at 1 year were 72% and 83% in lenvatinib-treated patients and 69% and 83% in DT-treated patients, respectively. In both selpercatinib-treated patients, the PFS at data cut-off was 10 months. No treatment-related deaths were observed.
Conclusion: S-TKIs permitted tailoring systemic treatment based on disease location, tumour volume and patient comorbidities, achieving satisfactory tolerance and outcomes in selected patients with an actionable driver mutation and with contraindications to angiogenesis inhibitors or candidates for redifferentiation therapy.
Keywords: advanced thyroid cancer; differentiated thyroid cancer; first-line treatment; selective kinase inhibitors; tyrosine kinase inhibitors.
Conflict of interest statement
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

