Proper 5'-3' cotranslational mRNA decay in yeast requires import of Xrn1 to the nucleus
- PMID: 39841709
- PMCID: PMC11753706
- DOI: 10.1371/journal.pone.0308195
Proper 5'-3' cotranslational mRNA decay in yeast requires import of Xrn1 to the nucleus
Abstract
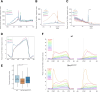
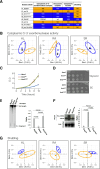
The budding yeast Xrn1 protein shuttles between the nucleus, where it stimulates transcription, and the cytoplasm, where it executes the major cytoplasmic mRNA decay. In the cytoplasm, apart from catalyzing 5'→3' decay onto non translated mRNAs, Xrn1 can follow the last translating ribosome to degrade the decapped mRNA template, a process known as "cotranslational mRNA decay". We have previously observed that the import of Xrn1 to the nucleus is required for efficient cytoplasmic mRNA decay. Here by using an Xrn1 mutant that cannot enter the nucleus, but is otherwise functional in ribonuclease activity, we show that nuclear import is necessary for proper global cotranslational decay of mRNAs along coding regions and also affects degradation in the of 5' region of a large group of mRNAs, which comprise about 20% of the transcriptome. Furthermore, a principal component analysis of the genomic datasets of this mutant and other Xrn1 mutants also shows that lack of a cytoplasmic 5'→3' exoribonuclease is the primary cause of the physiological defects seen in a xrn1Δ mutant, but also suggests that Xrn1 import into the nucleus is necessary for its full in vivo functions.
Copyright: © 2025 Jordán-Pla et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
VP is a co-founder and shareholder of 3N Bio AB. All the other authors declare no conflict of interest. VP does not receive any salary from 3N Bio and the company did not play any role in this study. VP role in 3N Bio AB does not alter our adherence to PLOS ONE policies on sharing data and material.
Figures

References
-
- Chang JH, Xiang S, Tong L. 50–30 Exoribonucleases. In: Nicholson A. W. (Eds.) Ribonucleases. 2011. Vol. 7. Heidelberg: Springer, pp. 167–192.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

