A pan-orthohantavirus human lung xenograft mouse model and its utility for preclinical studies
- PMID: 39841788
- PMCID: PMC11774489
- DOI: 10.1371/journal.ppat.1012875
A pan-orthohantavirus human lung xenograft mouse model and its utility for preclinical studies
Abstract
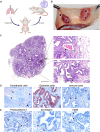
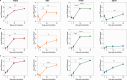
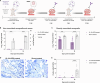
Orthohantaviruses are emerging zoonotic viruses that can infect humans via the respiratory tract. There is an unmet need for an in vivo model to study infection of different orthohantaviruses in physiologically relevant tissue and to assess the efficacy of novel pan-orthohantavirus countermeasures. Here, we describe the use of a human lung xenograft mouse model to study the permissiveness for different orthohantavirus species and to assess its utility for preclinical testing of therapeutics. Following infection of xenografted human lung tissues, distinct orthohantavirus species differentially replicated in the human lung and subsequently spread systemically. The different orthohantaviruses primarily targeted the endothelium, respiratory epithelium and macrophages in the human lung. A proof-of-concept preclinical study showed treatment of these mice with a virus neutralizing antibody could block Andes orthohantavirus infection and dissemination. This pan-orthohantavirus model will facilitate progress in the fundamental understanding of pathogenesis and virus-host interactions for orthohantaviruses. Furthermore, it is an invaluable tool for preclinical evaluation of novel candidate pan-orthohantavirus intervention strategies.
Copyright: © 2025 Rissmann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines, influenza virus vaccines and influenza virus therapeutics which list FK as co-inventor and of which several have been licensed. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, Castlevax, to develop SARS-CoV-2 vaccines. FK is co-founder and scientific advisory board member of Castlevax. FK has consulted for Merck, Curevac, Seqirus and Pfizer and is currently consulting for 3rd Rock Ventures, GSK, Gritstone and Avimex. The Krammer laboratory is also collaborating with Dynavax on influenza vaccine development. All other authors have no conflicts of interest to report.
Figures




References
-
- Pizarro E, Navarrete M, Mendez C, Zaror L, Mansilla C, Tapia M, et al. Immunocytochemical and ultrastructural evidence supporting that Andes hantavirus (ANDV) is transmitted person-to-person through the respiratory and/or salivary pathways. Front Microbiol. 2020;102992. doi: 10.3389/fmicb.2019.02992 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

