The deubiquitinase USP5 prevents accumulation of protein aggregates in cardiomyocytes
- PMID: 39841822
- PMCID: PMC11753375
- DOI: 10.1126/sciadv.ado3852
The deubiquitinase USP5 prevents accumulation of protein aggregates in cardiomyocytes
Abstract
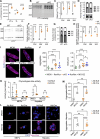
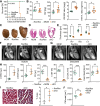
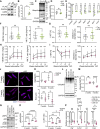
Protein homeostasis is crucial for maintaining cardiomyocyte (CM) function. Disruption of proteostasis results in accumulation of protein aggregates causing cardiac pathologies such as hypertrophy, dilated cardiomyopathy (DCM), and heart failure. Here, we identify ubiquitin-specific peptidase 5 (USP5) as a critical determinant of protein quality control (PQC) in CM. CM-specific loss of mUsp5 leads to the accumulation of polyubiquitin chains and protein aggregates, cardiac remodeling, and eventually DCM. USP5 interacts with key components of the proteostasis machinery, including PSMD14, and the absence of USP5 increases activity of the ubiquitin-proteasome system and autophagic flux in CMs. Cardiac-specific hUSP5 overexpression reduces pathological remodeling in pressure-overloaded mouse hearts and attenuates protein aggregate formation in titinopathy and desminopathy models. Since CMs from humans with end-stage DCM show lower USP5 levels and display accumulation of ubiquitinated protein aggregates, we hypothesize that therapeutically increased USP5 activity may reduce protein aggregates during DCM. Our findings demonstrate that USP5 is essential for ubiquitin turnover and proteostasis in mature CMs.
Figures







References
-
- Kim Y. E., Hipp M. S., Bracher A., Hayer-Hartl M., Hartl F. U., Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 (2013). - PubMed
-
- Kwon Y. T., Ciechanover A., The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem. Sci. 42, 873–886 (2017). - PubMed
-
- Chiti F., Dobson C. M., Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 86, 27–68 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

