Microplastics in the bloodstream can induce cerebral thrombosis by causing cell obstruction and lead to neurobehavioral abnormalities
- PMID: 39841831
- PMCID: PMC11753373
- DOI: 10.1126/sciadv.adr8243
Microplastics in the bloodstream can induce cerebral thrombosis by causing cell obstruction and lead to neurobehavioral abnormalities
Abstract
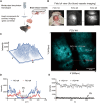
Human health is being threatened by environmental microplastic (MP) pollution. MPs were detected in the bloodstream and multiple tissues of humans, disrupting the regular physiological processes of organs. Nanoscale plastics can breach the blood-brain barrier, leading to neurotoxic effects. How MPs cause brain functional irregularities remains unclear. This work uses high-depth imaging techniques to investigate the MPs within the brain in vivo. We show that circulating MPs are phagocytosed and lead these cells to obstruction in the capillaries of the brain cortex. These blockages as thrombus formation cause reduced blood flow and neurological abnormalities in mice. Our data reveal a mechanism by which MPs disrupt tissue function indirectly through regulation of cell obstruction and interference with local blood circulation, rather than direct tissue penetration. This revelation offers a lens through which to comprehend the toxicological implications of MPs that invade the bloodstream.
Figures







References
-
- Banerjee A., Shelver W. L., Micro- and nanoplastic induced cellular toxicity in mammals: A review. Sci. Total Environ. 755, 142518 (2021). - PubMed
-
- Huang W., Song B., Liang J., Niu Q., Zeng G., Shen M., Deng J., Luo Y., Wen X., Zhang Y., Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 405, 124187 (2021). - PubMed
-
- Law K. L., Narayan R., Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 7, 104–116 (2022).
-
- Jambeck J. R., Geyer R., Wilcox C., Siegler T. R., Perryman M., Andrady A., Narayan R., Law K. L., Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

