Mitochondria complex III-generated superoxide is essential for IL-10 secretion in macrophages
- PMID: 39841842
- PMCID: PMC11753406
- DOI: 10.1126/sciadv.adu4369
Mitochondria complex III-generated superoxide is essential for IL-10 secretion in macrophages
Abstract
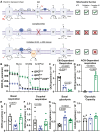
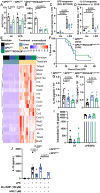
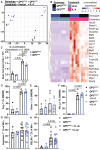
Mitochondrial electron transport chain (ETC) function modulates macrophage biology; however, mechanisms underlying mitochondria ETC control of macrophage immune responses are not fully understood. Here, we report that mutant mice with mitochondria ETC complex III (CIII)-deficient macrophages exhibit increased susceptibility to influenza A virus (IAV) and LPS-induced endotoxic shock. Cultured bone marrow-derived macrophages (BMDMs) isolated from these mitochondria CIII-deficient mice released less IL-10 than controls following TLR3 or TLR4 stimulation. Unexpectedly, restoring mitochondrial respiration without generating superoxide using alternative oxidase (AOX) was not sufficient to reverse LPS-induced endotoxic shock susceptibility or restore IL-10 release. However, activation of protein kinase A (PKA) rescued IL-10 release in mitochondria CIII-deficient BMDMs following LPS stimulation. In addition, mitochondria CIII deficiency did not affect BMDM responses to interleukin-4 (IL-4) stimulation. Thus, our results highlight the essential role of mitochondria CIII-generated superoxide in the release of anti-inflammatory IL-10 in response to TLR stimulation.
Figures


References
-
- Jha A. K., Huang S. C.-C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K. M., Ashall J., Everts B., Pearce E. J., Driggers E. M., Artyomov M. N., Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

