Beyond In Vivo, Pharmaceutical Molecule Production in Cell-Free Systems and the Use of Noncanonical Amino Acids Therein
- PMID: 39841856
- PMCID: PMC11826901
- DOI: 10.1021/acs.chemrev.4c00126
Beyond In Vivo, Pharmaceutical Molecule Production in Cell-Free Systems and the Use of Noncanonical Amino Acids Therein
Abstract
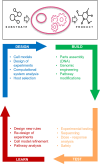
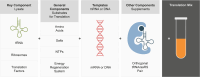
Throughout history, we have looked to nature to discover and copy pharmaceutical solutions to prevent and heal diseases. Due to the advances in metabolic engineering and the production of pharmaceutical proteins in different host cells, we have moved from mimicking nature to the delicate engineering of cells and proteins. We can now produce novel drug molecules, which are fusions of small chemical drugs and proteins. Currently we are at the brink of yet another step to venture beyond nature's border with the use of unnatural amino acids and manufacturing without the use of living cells using cell-free systems. In this review, we summarize the progress and limitations of the last decades in the development of pharmaceutical protein development, production in cells, and cell-free systems. We also discuss possible future directions of the field.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
[Progress of cell-free protein synthesis system and its applications in pharmaceutical engineering - A review].Wei Sheng Wu Xue Bao. 2016 Mar 4;56(3):530-42. Wei Sheng Wu Xue Bao. 2016. PMID: 27382794 Review. Chinese.
-
Unnatural amino acids: production and biotechnological potential.World J Microbiol Biotechnol. 2019 Apr 8;35(4):67. doi: 10.1007/s11274-019-2642-9. World J Microbiol Biotechnol. 2019. PMID: 30963257 Review.
-
Protein and cellular engineering with unnatural amino acids.Biotechnol Prog. 2007 Jan-Feb;23(1):28-31. doi: 10.1021/bp060369d. Biotechnol Prog. 2007. PMID: 17269666 Review.
-
In vitro suppression of two different stop codons.Biotechnol Bioeng. 2017 May;114(5):1065-1073. doi: 10.1002/bit.26226. Epub 2016 Nov 28. Biotechnol Bioeng. 2017. PMID: 27882539
-
Chemocavity: specific concavity in protein reserved for the binding of biologically functional small molecules.J Chem Inf Model. 2008 Aug;48(8):1679-85. doi: 10.1021/ci800113c. Epub 2008 Jul 22. J Chem Inf Model. 2008. PMID: 18642867
Cited by
-
Cell-free protein synthesis platforms for accelerating drug discovery.Biotechnol Notes. 2025 Feb 19;6:126-132. doi: 10.1016/j.biotno.2025.02.001. eCollection 2025. Biotechnol Notes. 2025. PMID: 40123759 Free PMC article. Review.
-
Effect of glycosylation on protein folding: From biological roles to chemical protein synthesis.iScience. 2025 May 8;28(6):112605. doi: 10.1016/j.isci.2025.112605. eCollection 2025 Jun 20. iScience. 2025. PMID: 40502693 Free PMC article. Review.
References
-
- Wolfe N. D.; Diamond J.. ORIGINS OF MAJOR HUMAN INFECTIOUS DISEASES. In Institute of Medicine (US). Improving Food Safety Through a One Health Approach: Workshop Summary; National Academies Press (US),:Washington (DC), 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

